Abstract
Background and aims
Nickel (Ni) deficiency has been reported to occur in soybean (Glycine max) grown on leached tropical soils in Brazil. We aimed to determine whether an internal or external Ni supply can compensate for low Ni within the seed by assessing whether the amount of Ni in the seed whether the foliar-application of aqueous NiSO4 influenced the uptake of Ni by the leaf, the nutritional status of the plant, urease activity and growth.
Methods
We used Ni-depleted seeds (<0.35 μg Ni per g) and Ni-sufficient seeds (11.1 μg Ni g−1) for hydroponic experiments. Seedlings were grown either with or without an external Ni supply (0 or 0.85 μM Ni in nutrient solution) and either with or without an internal Ni supply (with or cotyledons removed). In addition, we used synchrotron-based micro-X-ray fluorescence analysis to examine the distribution of foliar-applied Ni (50 and 100 mg L-1).
Key results
Leaf Ni concentration and urease activity were both enhanced by increasing either the internal (cotyledon seed store) or external (solution) Ni supply. In addition, plants derived from Ni-depleted seed that received external Ni supply had 9.2% higher biomass relative to plants derived from Ni-sufficient seeds which received Ni. When foliar-applied, Ni accumulated in the pedicles of the trichomes within 15 minutes of application, and then moved to the vascular bundles before dispersing further into tissues within 3 hours.
Conclusions
Trichomes are an important pathway for foliar Ni absorption in soybean, but there are still major knowledge gaps our understanding of the physiological function of trichomes in the uptake of metal ions from foliar micro-nutrient treatments.
Similar content being viewed by others
Introduction
Nickel (Ni) is a cofactor of the urease enzyme (EC 3.5.1.5, urea amidohydrolase) which contains two Ni atoms at the active site (Ciurli 2001). These Ni atoms are therefore directly involved in nitrogen (N) metabolism and improving Ni nutrition could increase crop yield by upregulating urea decomposition and N-fixing enzyme activity. Urease is responsible for the hydrolysis of urea and ureides, releasing two molecules of ammonia and one of carbon dioxide (Dixon et al. 1975; Eskew et al. 1983; Witte 2011; Polacco et al. 2013; Ohyama et al. 2017; Todd et al. 2006). Subsequent experimentation established the essentiality of Ni for non-legumes grown with ammonium and nitrate supply (Brown et al. 1987). Nickel deficient plants accumulate toxic concentrations of urea in their leaf tips, but Ni addition prevents toxicity (Eskew et al. 1983; Shimada and Ando 1980; Brown et al. 1987; Rutter 2005). Nickel deficiency causes necrosis at the tip of the leaves due to disrupted of ureide catabolism and affects other metabolism process such as amino acid and the citric acid cycle (Bai et al. 2006; Wood 2006; Freitas et al. 2018).
Soybean is one of the most important agricultural crops worldwide, being a major source of cattle feed, products for human consumption and vegetable oil (Food Agriculture Organization of the United Nations 2017). It is often cultivated on leached tropical soils (especially in Brazil) with low Ni availability and with strong Ni adsorption, which can cause a hidden Ni deficiency potentially to occur (Barcelos et al. 2018; Freitas et al. 2018; Macedo et al. 2020). Under such circumstances, plants do not reach their maximum growth potential despite a lack of visible deficiency symptoms (Dalton et al. 1985; Dabkowska-Naskret et al. 2014; Freitas et al. 2018, 2019; Jaworska et al. 2013; Licht et al. 2006; Morrison et al. 2009; Roca et al. 2008; Rodak et al. 2015). In addition, for soybean plants with insufficient Ni, urease activity decreases resulting in the accumulation of urea-related N compounds, causing toxicity and reduced plant growth (Eskew et al. 1983; Brown et al. 1990; Krogmeier et al. 1991; Lavres et al. 2016; Reis et al. 2017). Remediating Ni deficiency in soybean can be achieved by the application of Ni, with the Ni either applied directly on the seeds at planting, or by Ni foliar application of mature plants (Hosseini and Khoshgoftarmanesh 2013), or by soil Ni fertilization (Macedo et al. 2016, 2020; Rodak et al. 2021). In this study, we have a particular interest in examining how Ni is distributed and translocated in soybean leaves in response to foliar application of Ni, which has been shown to increase urease activity in soybean plants (Ojeda-Barrios et al. 2016). Additionally, Ni foliar application can avert toxic urea accumulation in leaf tissue, and glyphosate drift, which are the toxic symptoms caused by the non-hydrolysis of urea (Eskew et al. 1984; Kutman et al. 2013; Kutman et al. 2014). However, despite the importance of foliar fertilization, including for Ni, many questions remain about how micro-nutrients move into the main organs following their foliar application (Fernández et al. 2017; Li et al. 2017). The lack of knowledge of physiological processes involved in the foliar uptake of applied micro-nutrients via the foliage impedes efforts to increase the success of foliar micro-nutrient fertilizers in plants (Fernández et al. 2013; 2021). Furthermore, the coupling of biochemical analyses and imaging coupled assay can be used as complementary techniques to evaluate the nutritional status of plant together with traditional methods of foliar diagnosis (Oliveira et al. 2021).
Understanding the process of Ni absorbtion following its application via the seeds, leaves or roots is the first step to determine effective strategies aiming to improve plant nutrient management (Freitas et al. 2020; Oliveira et al. 2021, 2022). The use of X-ray fluorescence (XRF) techniques is an effective, non-destructive and efficient method for evaluation of the spatial distribution of elements in plant tissues. Synchrotron-based micro-X-ray fluorescence (μXRF) analysis is a powerful technique that allows for in situ analyses of the tissue-scale spatial distribution of a wide-range of different elements in plants (Kopittke et al. 2018; van der Ent et al. 2018; 2019). There are still only a limited number of investigations that have examined changes in nutritional distribution over time (at spatiotemporal scale) in living plants using X-ray elemental techniques at synchrotron facilities (Scheckel et al. 2004; Lombi et al. 2011; Blamey et al. 2018; Li et al. 2019), a subset of these having focussed on the toxic effects of Mn in soybean, cowpea and sunflower (Blamey et al. 2018; Doolette et al. 2018). Previous experiments examining the foliar application of Zn fertilizers in sunflower have shown the foliar Zn absorption occurs mainly via trichomes, with Zn specifically accumulating within trichomes in ≤15 min, with the stomatal pathway not the only way to nutrient diffusion and further absorption (Li et al. 2019). Furthermore, it has been noted that substantial quantities of fluorescent and ionic tracers can be absorbed and translocated by trichomes, probably because they do not have a cuticle but are surrounded by a pectin-rich cell wall layer (Schreel et al. 2020). However, there is still a lack of information at spatiotemporal scale of Ni absorption and translocation after foliar application and its transference into to the main organs to fulfil the nutritional requirements of plants. Therefore, current advances in plant nutritional status evaluation and experimental methods open the path to unravel the importance and interconnectedness of the processes of micro-nutrient uptake by plants.
The present study aimed to: (i) establish whether an internal or external Ni supply can compensate for low Ni within the seed; (ii) determine the influence of seed stored Ni at tissue-scale range of Ni-concentration, time-scale of leaf applied Ni-distribution and translocation, and upregulation of urease activity.
Materials and methods
Overall experimental design
Three different experiments were carried out to understand the effects of Ni distribution and translocation on soybean growth. The first hydroponics experiment grew soybean plants to maturity in Ni-purified hydroponics culture to produce Ni-depleted seeds. The second experiment used plants grown from Ni-sufficient seeds to examine the lateral distribution of foliar applied Ni (as aqueous NiSO4) in situ within hydrated intact soybean leaves using synchrotron-based μXRF analysis. The third experiment examined the effect of seed Ni concentration, seed Ni internal contribution (based on the presence or absence of cotyledons), and external supply of Ni on biomass, tissue elemental concentrations and urease activity. This third experiment used the original Ni-sufficient seeds (11.1 μg Ni per g) plus the Ni-depleted seeds (<0.35 μg Ni per g) to grow plants which were then dosed with or without Ni at 0.85 μM in solution and on which cotyledons were either kept attached or excised. The concentration of Ni has been quantified in soybean grains through prior studies conducted in the United States, revealing a range from 0.35 to 29 μg Ni per g) (Wolnik et al. 1983).
Experiment 1: Hydroponics culture to generate nickel-depleted seeds
Soybean plants were grown in nutrient solution in controlled conditions at The University of Queensland (St Lucia, Australia) from October 2019 to February 2020. Nickel-sufficient seeds (Glycine max (L.) Merr. cv Bunya – containing 11.1 μg Ni per g) were germinated on a moistened fine perlite/vermiculite mixture at 26 °C. After one week, corresponding to the vegetative phenological stage of cotyledons, the seedlings were transferred to four 30 L containers containing nutrient solution. The lids of the containers had four holes in which the seedlings were inserted in foam baskets, with one seedling per hole. The cotyledons were excised upon transfer in order to remove the Ni-store in the seed. The nutrient solution was continuously aerated and kept at room temperature (22 °C) with a relative air humidity of 60–70 RH%, with light provided using high-intensity full-spectrum LED lighting (Black Dog LED, PhytoMAX-2400, 365–750 nm, photon flux density of 1000 μmol m−2 s−1) for 12 h d−1. The nutrient solution contained: total 4.5 mM K+ (as KNO3 + other K-salts listed), 2.5 mM Ca2+ (as Ca(NO3)2), 1 mM HPO42− as K2HPO4), 2 mM Mg2+ (as MgSO4), 1 μM Cl− (as KCl), 2 μM B+ (as H3BO3), 1 μM Mn2+ (as MnSO4), 2 μM Zn2+ (as ZnSO4), 3 μM Cu2+ (as CuSO4), 4 μM MoO42− (as Na2MoO4), and total 5 mM NO3− (from various nitrate salts listed). During the culture period the soybean plants did not develop biological nitrogen fixation (as judged by visual inspection of roots which did not have nodulation). The Fe-source was Fe-diethylenetriaminepentaacetic acid (FeDTPA, 40 μM Fe) as earlier tests with Fe-hydroxybenzyl ethylenediamine (FeHBED) showed that Fe uptake was limited, even at 100 μM Fe. Nutrient solutions were fully changed twice a week and the nutrient solution pH was maintained at pH 5.8 by daily additions of 0.01 M KOH or 0.01 M HNO3 solution as required. To achieve Ni-limiting conditions in the nutrient solutions, high-purity (AR trace grade) chemical reagents were used, and the solutions were purified as follows: the macro-element concentrate was purified using Chelex 100 resin (Bio Rad iminodiacetate type resin) to remove polyvalent metal ions (Fe2/3+, Mn2+, Ni2+, Cu2+, Zn2+). The micro-nutrient concentrate was purified using a Ni-specific resin (Eichrom Nickel Resin based on dimethylglyoxime, DMG) to selectively remove Ni2+ ions. Measured concentrations of Ni in the nutrient solution were < 0.001 mg L−1 (this equals to 0.02 μM Ni) as determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The plants were grown to complete physiological maturity after 110 d (which corresponds to the R7 phenological stage). The Ni-depleted seeds were harvested when plants senesced completely, with these Ni-depleted seeds found to contain <0.35 μg Ni per g, compared to the value of 11.1 μg Ni per g for the original Ni-sufficient seeds.
Scanning electron microscopy (SEM)
Plant specimens at the R1 stage (42 d) from Exp #1 were freeze-dried for SEM analysis by rapid freezing against a solid metal block cooled by liquid nitrogen (−196 °C). Then the cooled block with samples was loaded into a lyophilizer (Thermoline), vacuum-pumped and set to −85 °C. The lyophilisation was carried out gradually by increasing in 5 °C steps throughout 3 days until it reached room temperature. Afterward, the specimens were then sputter-coated with carbon (∼25 nm) and fixed on stubs. The specimens were imaged using a Hitachi SU3500 instrument at 100–1000× magnification at 4–5 kV with secondary electron (SE) returns.
Experiment 2: Synchrotron-based μXRF analysis
This experiment aimed to examine the distribution of Ni within the plant tissue after foliar Ni application treatments. Plants were grown from (original) Ni-sufficient seeds in Ni-purified nutrient solution, as described for Exp #1, until they reached 14-days old – equivalent to phenological stage V3. The Ni foliar fertilizer was prepared at two concentrations: 50 and 100 mg L-1 (i.e., 0.85 and 1.7 mM Ni) from NiSO4. The pH of the solutions ranged from pH 5.4 to pH 5.1 for the Ni treatments of 50 and 100 mg L-1. The solution also contained 0.5 μL of Silwet® L-77 (trisiloxane-based adjuvant) per 10 mL of solution as a non-ionic surfactant/wetting agent. We first conducted a time series experiment to investigate the Ni uptake process using intact, hydrated young leaves (14 d old) for which NiSO4(aq) foliar application was used. Specifically, we investigated several foliar application times: 0 min (control), 45 min. and 3 hrs. for 50 mg L-1 of Ni, and 0 min (control), 15 min., 25 min., 45 min. and 3 hrs for 100 mg L-1 Ni. The initial Ni application rates were based on preliminary tests, while for the higher application rate, we opted for a more extensive range of time intervals to enhance the precision of information regarding the uptake process. For each of these treatments, the adaxial surface of the soybean leaves received two 5-μL droplets of NiSO4 solution and the entire leaf (whilst still attached to the plant) was placed in a Petri dish containing a hole in the side wall with the stem passing through the hole in the side. To maintain the humidity inside the Petri dish moist filter paper was added to the bottom of the plate. The environment within the Petri dish was maintained at 30 °C and the relative humidity above 98% RH. In the experimental period, the LED lighting was turned on throughout the incubation period and the droplets in the leaf surface were carefully maintained as a liquid (i.e., the droplets did not dry out). Subsequently, the leaves were excised from the plant and rinsed using tap water (Supplementary Fig. 2). For each sample, the washed leaf was carefully blotted dry and sandwiched on a sample holder between two stretched sheets of Ultralene thin-film (4 μm thick). Samples were then immediately analyzed using μXRF at the X-ray fluorescence microscopy (XFM) beamline of the Australian Synchrotron.
The XFM beamline, with its 384-element Maia detector system in a backscatter geometry, was used to collect X-ray fluorescence emitted by the specimen (Howard et al. 2020). An incident energy of 12.9 keV with a total photon flux of approximately 1.5 × 109 photons s−1 was used. First an overview scan was carried out to obtain a comparatively fast map of the leaf to select areas for the detailed scans. The detailed scans were typically approx. 4 × 5 mm in size, used a step size of 10 μm, had a dwell of 5 ms, and took approx. 75 min. to complete. An important concern that needs be considered is that fresh hydrated specimens may be affected by radiation-induced damage from the μXRF analysis. To avoid this type of damage in the hydrated plant tissues, the dose was limited to <4.1 kGy (Jones et al. 2020). The Dynamic Analysis method was used to analyse the synchrotron μXRF event stream (Ryan and Jamieson 1993; Ryan 2000) as implemented in GeoPIXE (Ryan et al. 1990, 1995).
Experiment 3: Nickel dosing to plants grown from Ni-depleted and Ni-sufficient seeds
We used two types of seeds: (i) Ni-depleted seeds described above; and (ii) the original Ni-sufficient seeds. The experiment consisted of a total of eight treatments, with two types of seeds (Ni-depleted and Ni-sufficient seeds), two solution Ni concentrations: 0 and 0.85 μM Ni (supplied in the form of NiSO4) (Reis et al. 2017; De Carvalho Braga Levy et al. 2019), and two cotyledon treatments (cotyledons attached, or cotyledons removed after germination). This experiment aimed to examine the effects of Ni concentration on plants grown from either Ni-sufficient or Ni-depleted seeds and the effect of cotyledon Ni-store removal. Each treatment condition (two types of Ni seeds × cotyledon attached or removed × 0 or 0.85 μM Ni) had four biological replicates. Free metal ion activities (−log10 values) were determined using GEOCHEM-EZ software (Shaff et al. 2010) of the full nutrient solution formulation in the 0.85 μM Ni treatment and the pNi2+ = 11.4. The two types of seeds were placed on moistened fine perlite/vermiculite mixture for one week. The emerged seedlings were then transferred to 20 L containers filled with the nutrient solution described above, with the lids having six holes for each replicate. The nutrient solution was the same as outlined previously, with solutions changed once per week and maintained at pH 5.8. The soybean plants were harvested at phenological stage V4 (fourth trifoliate; Fehr et al. 1971). The third expanded leaf (from the top) was collected for analysis of urease activity (as described in detail below). At the end of the experiment, roots, tap root, stem, young (fourth and younger trifoliolate leaves) and old (primary and first two trifoliolate leaves) leaves were separately collected for acid digestion and ICP-AES. Wet and dry weights of the various plant fractions were also determined.
Urease activity measurements
The urease activity was analysed in vivo twice (20 d – V3 and 27 d – V4 after germination) by an adaptation of the method described by Hogan et al. (1983). 8 mL of NaH2PO4 buffer urea at pH 7.4 was prepared to place the plant tissues from Exp #3 (0.2 g of fresh leaves, cut into slices with a width 1 mm). The plants were incubated in the buffer for 3 h at 30 °C and mixed every 5 min. in a Falcon tube of 15 mL were added 0.5 mL of the extract obtained after incubation, 2.5 mL of Reagent 1 (phenol 0.1 mol L−1, sodium nitroprusside – 50 mg) and 2.5 mL of a solution containing sodium hydroxide 0.125 mol L−1; sodium dihydrogen phosphate dodecahydrate 0.15 mol L−1; and sodium hypochlorite 3% Cl2. Then, the tubes were closed with lids (so as to not lose the NH3) and put into a water bath at 37 °C for 35 min. Then, the N-NH4-concentration was measured using a spectrophotometer at 625 nm (Agilent Technologies, Santa Clara, CA, USA). The amount of ammonium (NH4+) produced by the reaction was used to measure urease activity, and the values were compared with a standard curve, determined using NH4Cl.
Elemental analysis of plant tissues
All combinations of seeds × Ni solution concentration × Ni foliar treatments from plants of Exp #3 were analyzed for the elemental analysis. In addition, seeds, cotyledons, and unripe pods from plants of Exp #1 were analysed. Parts of the plant material samples (old leaves, young leaves, tap root and stem from each treatment) were oven dried at 60 °C for 3 d. The soybean tissues were powdered at 15,000 rpm in an IKA Tube Mill. The ground samples were weighted using a precision scale (to 100 ± 5 mg) and placed in 6 mL falcon tubes. Subsequently, 2 mL of AR-grade HNO3 (70%) was added to these samples and left overnight for pre-digestion and the samples were then fully digested in a block heater (Thermo Scientific™ digital dry bath) for 1 hr at 70 °C followed by 1 hr at 125 °C and made to volume (10 mL) with ultrapure water (Millipore 18.2 MΩ·cm at 25 °C). The digestates were finally analysed by ICP-AES (Thermo Scientific iCAP 7400 instrument) focusing on the macro-elements: Na, Mg, Al, P, S, K, Ca, and the trace-elements: Cr, Mn, Fe, Co, Ni, Cu, Zn in radial and axial modes depending on the element and expected analyte concentration. In-line internal addition standardization using yttrium was used to compensate for matrix-based effects. Matrix blanks and SRMs were included (NIST 1515 Apple Leaves). The limit of detection for Ni (on the basis of 100 mg of sample in 10 mL acid digestion solution) was 0.35 μg g−1 and values below this were considered as not quantifiable and not used.
Statistical analysis
Data analysis was performed with R Development Core team version 4.3.1 (2023). The data were submitted to analysis of variance by the F-test using the package lme4 (Bates et al. 2015), dplyr (Wickham et al. 2022) and tidyr (Wickham et al. 2023). Data were visualized using ggplot2 package (Wickham 2016). When the effect was significant, we applied the Tukey test to compare the effects. In all analysis, the level of significance was 5%. The data were subjected to two-way ANOVA to determine the effects of main effects: type of seeds (Ni depleted and Ni sufficient) and presence or absence of cotyledons and the interaction: type of seeds versus cotyledons factor.
Results
Nickel depletion in hydroponics culture
First, plants were grown to maturity (phenological stage R7-110d) under Ni-limiting conditions in hydroponics to generate Ni deficient seeds for the subsequent Ni dosing experiment (Exp #3). The mature plants in Exp #1 displayed mild Ni deficiency symptoms, including foliar chlorosis, especially at the leaf tips and margins, and some seed pods were infertile (Supplementary Fig. 2). The original seeds contained 11.1 μg g−1 Ni, whereas the Ni depleted-seeds produced in Exp #1 contained <0.35 μg g−1 Ni (Table 1). We examined the leaves from plants grown in Exp #1 using SEM, with this being important for understanding the foliar absorption of Ni from NiSO4.6H2O fertilizer subsequently in Exp #3. Two types of trichomes were found on the adaxial surface of soybean (Fig. 1a): non-glandular trichomes (multicellular, thick walled and non-secretory) and glandular trichomes (multicellular and secretory). The glandular trichomes (Fig. 1b) are comparatively short structures and were highly vacuolated. They typically consisted of an epidermal cell and around four or five stalk cells with one small cell on the apex. The external morphology of the glandular trichomes had a series of strong linear cells separated by periclinal cross walls. The non-glandular trichomes (Fig. 1c) were empty, comprising of a multicellular base and one long stalk cell, namely the pedicle.
Scanning electron microscopy (SEM) images of trichomes on the adaxial surface of soybean leaves at the R1 stage from plants originating from Exp #1. In figs. (A-B), an overview of the soybean leaf surface reveals diverse trichomes types; (C-D) detailed image of the trichomes: glandular trichomes (GT) and non-glandular trichomes (NGT), as illustrated in figs. C and D, respectively. Further insight into the trichome is provided in (E-F)
Synchrotron μXRF analysis of soybean seeds
The spatial distribution of elements in whole soybean seeds (original stock with 11.1 μg g−1 bulk Ni) was analysed by synchrotron-based μXRF. Supplementary Fig. 3 shows the distribution of Ca, K, Fe, Zn, Mn, and Ni in the seeds. Calcium was localised around the seed coat (average of 1.5 μg g−1), with hotspots in parts of the hilum (0.3 μg g−1). Potassium was localised in the endosperm of the seed and was very low in the radicle (0.2 μg g−1), while there were some regions of the seed coat with higher prevailing K concentrations (1.4 μg g−1) (Supplementary Fig. 3). Similar to Ca, Fe was localised in the seed coat (0.15 μg g−1) and in hotspots at the tip of the radicle (0.5 μg g−1). Zinc had a more homogeneous distribution, and it was relatively uniform localised throughout the seed (in the endosperm and seed coat), however in the radicle Zn was more enriched up to 250 μg g−1 (Supplementary Fig. 3). Prevailing Mn concentrations were extremely low, although there were some hotspots at the margin of the radicle (up to 200 μg g−1), while other parts of the seed have approximately 50 μg g−1 Mn. Nickel was mainly localised in the seed coat, being very low throughout the rest of the seed, with an average of 40 to 20 μg g−1. However, there were areas of hotspots in the seed coat, where the concentration of Ni was around 80 μg g−1.
Synchrotron μXRF analysis of uptake and redistribution of foliar applied nickel
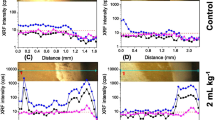
Changes in the spatiotemporal distribution of Ni (and other elements) were examined in situ using fresh hydrated soybean leaf tissues at different stages from Exp #2 following exposure to foliar Ni application at 50 and 100 mg L-1 in 5 μL droplets (as NiSO4 solution) after 15 min and after 3 hrs. Rapid survey scans in soybean leaves were used to select the areas of interest, with these demonstrating that within 15 min. the Ni had already relocated across the leaf surface and was present inside the leaf tissues, mainly around the trichomes (Fig. 2) and tissue Ni concentrations increased as the exposure time increased. After 15 min., Ni was observed to accumulate in the pedicles of the trichomes with some Ni moving toward the rest of the leaf through the veins; movement continued in the 3-hr study. Nickel was strongly concentrated in the trichome pedicles and Mn was strongly concentrated in the trichome bases, respectively. Figure 2 shows the distribution of Ni and Mn in a hydrated leaf and panels show trichomes after 15- and 25-min. exposure to 100 mg L-1 NiSO4 solution; Ni was clearly present in the pedicles of the trichome, whilst Mn was localised in the bases of the trichomes. Figure 2 shows foliar application of Ni at 45 min. and 3 hrs. exposure to NiSO4 solution which suggests that the Ni had rapidly translocated away from the site of application. The overall patterns were very similar after application of 50 mg L-1 Ni (Fig. 3) compared to 100 mg L-1 Ni. Detailed scans were then used to examine Ni distribution more closely around trichomes after foliar application. High concentrations of Ni accumulated in the pedicles of trichomes, suggesting that the most likely pathway of Ni entry is though the trichomes, as the plants were grown in a culture system devoid of Ni. As such, it unlikely the Ni deposition was delivered by leaf cells or from root uptake to the trichomes (Fig. 3). Using tri-colour maps of Ca-Ni-Mn (Fig. 4) it is evident that Ni and Mn concentrate near the trichomes, whereas Ca is more widespread throughout the tissues. Zinc follows a similar trend as Mn, locating mainly in the trichome bases (Fig. 4). Detailed μXRF scans (Fig. 4) of leaf surfaces after foliar application of Ni on soybean between 25 and 45 min. showed that 15 min. after foliar application, Ni was present only in the pedicles of the trichomes, whilst Mn was concentrated only in the bases of trichomes.
Synchrotron μXRF elemental maps showing the distribution of Ni and Mn after foliar application of 100 mg L−1 at 15, 25, 45 min. and 3 hrs. time intervals in soybean leaf. The concentrations are in wt% for Mn in the second panel and μg g−1 for Ni and Mn in all other panels with brighter colours corresponding to higher prevailing elemental concentrations. A-F have the same colour scale, where brighter colours correspond to higher Ni and Mn concentrations, as shown in the key. The scale bar applies for all the figures. Droplets were left on the left surface for 15 min., 25 min., 45 min. and 3 hrs.
Synchrotron μXRF elemental maps showing the distribution of Ni and Mn. Images show areas where droplets of 50 mg L−1 as NiSO4 were deposited on the leaves of soybean at 45 min. and 3 hrs time intervals. The concentrations are in μg g−1 with brighter colours corresponding to higher prevailing elemental concentrations. The figs. A-F have the same colour scale, where brighter colours correspond to higher Ni and Mn concentrations, as shown in the key. Fig. E-F shows high resolution of the trichomes structures. The arrows indicate absence of Mn in the presence of Ni
Synchrotron μXRF elemental maps showing the distribution of Ni, Mn, and Zn. The tricolour image shows Ca in red, Mn in green and Ni in blue. Fig. A and C show detail of trichomes at foliar Ni application doses of 100 mg L−1 at 45 min. and 3 hrs., respectively. Fig. B and D show the details of trichome base for Mn, and Zn, respectively. The concentrations are in μg g−1 where brighter colours corresponding to higher prevailing elemental concentrations
Effect of nickel supply on tissue concentrations and urease activity
Plants were cultured in (Ni-purified) hydroponics solution using the original (Ni-sufficient) and Ni-depleted seeds for a period of three weeks (V4 phenological stage) (Exp #3). Nickel was supplied in solution at 0 (control) or 0.85 μM Ni dose rate, and at the conclusion of the experiment, root and shoot biomass were determined, urease activity measured, and elemental concentrations determined in the different plant parts. The Ni concentration in soybean tissues (root, stem, young, old leaves, all leaves, and whole plant) was compared in plants where the cotyledons were either removed or where they were retained, both without external Ni application (control) or with additional Ni (0.85 μM) (Fig. 5). The presence of the cotyledons, even though they contained very low Ni (<0.35 μg g−1 of Ni – Table 1), was sufficient to increase the Ni concentration in soybean tissues compared to the plants that had their cotyledons removed, even without external Ni supply. Plants grown from Ni sufficient seeds stock (Table 1) shows that plants with attached cotyledons had an increase of 2.2-fold of Ni concentration compared to plants with removed cotyledons without external Ni application.
Box plot of Ni concentrations in organ-tissue scale of the soybean plants grown from two types of seeds (Ni-depleted seeds: red- and Ni-sufficient seeds: black), with (“with”) or cotyledon removed (“Removed”) and two Ni treatments: control and 0.85 μM Ni in different parts of soybean plant: (a and c) stem; (b and d) roots; (e and g) old leaves; (f and h) young leaves; (i and k) all leaves and (j and l) whole plant. Boxes with different letters indicate significant differences according to least difference (Tukey) test P < 0.05. ** indicates that there was interaction between the factors according to least difference (Coty*Seed)
The average tissue Ni concentration was higher when the plants were grown in solutions containing 0.85 μM Ni compared to the control with 0 μM Ni (Fig. 5). The Ni dose increased the Ni concentration in the Ni-depleted seeds 3.12-fold with the cotyledons attached and 3.13-fold in the absence of the cotyledons in the whole plant. For the plants grown from Ni-sufficient seeds, the external supply of Ni increased Ni concentrations 3.78-fold in plants with cotyledons and 7.12-fold in plants without the cotyledons. Plants with cotyledons attached that received Ni grown from Ni sufficient seeds, had a higher concentration of Ni than those grown without cotyledons (Fig. 5), an average of 1.18-fold in the whole plant (stem, roots, old and young leaves) (Fig. 5i). Plants with cotyledons attached grown from Ni-depleted seeds also had a higher Ni concentration (average of 1.09-fold higher) compared to plants with the cotyledon removed from the whole plant. The young leaves from plants grown without cotyledons had an average of 1.61-fold higher Ni compared to those with cotyledons (Fig. 5g).
Figure 6 shows the biomass in different soybean parts: stem, roots, old leaves, and young leaves after different treatments. The plants grown from Ni-sufficient seeds in solution without Ni (0 μM) and those where the cotyledon was attached both had increased biomass compared to the other treatments (Fig. 6k). The plants grown from Ni-depleted seeds whose cotyledons were removed had a lower biomass compared to the plants with cotyledons still attached, being an average of 31% lower. Therefore, seedling development was affected by the presence of cotyledons even in a Ni-free solution. The plants with the cotyledons attached that received Ni (0.85 μM) (Fig. 6) also resulted in an increase of the biomass in the stem and old leaves tissues compared to the plants with cotyledons removed. Additionally, Ni depleted seeds that received Ni had increased biomass (9.2% higher) relative to plants grown from Ni sufficient seeds which received Ni (Fig. 6k).
Box plot of dry weight (g) of soybean parts: stem; roots; old leaves; young leaves; all leaves and whole plant. Plants were grown from two types of seeds (Ni-depleted seeds – red- and Ni-sufficient seeds-black), with (“with”) or cotyledon removed (“Removed”) and two Ni treatments: control 0 μM Ni and 0.85 μM Ni. Boxes with different letters indicate significant differences according to least difference (Tukey) test P < 0.05. ** indicates that there was interaction between the factors according to least difference (Coty*Seed)
Urease activity in nickel treated plants
The urease activity (Fig. 7) was influenced by the different seed types (Ni-depleted and Ni-sufficient) as well as the Ni treatment (0 and 0.85 μM Ni). The addition of 0.85 μM Ni to the solution increased urease activity in the plants grown from both the Ni-depleted and Ni-sufficient seeds. In the plants grown from Ni-depleted seeds, urease activity increased ca. 260% at 0.85 μM Ni. For plants grown from Ni-sufficient seeds, the urease activity tended to be slightly higher than in plants grown from Ni-depleted seeds, especially in the control (0 μM Ni). The presence of the cotyledons in the Ni-sufficient seeds also increased the urease activity even without external Ni supply, increasing from 1.9 (removed cotyledons) to 4.6 N NH4+ g−1 h−1. Therefore, urease activity was enhanced by higher levels of Ni in the plants.
Urease activity in leaves from plants originating from Exp #3 (grown from Ni depleted seeds and Ni sufficient seeds), with two Ni treatments Ni (control - 0 and 0.85 μM Ni) after 27 days germination). Different letters represent significant differences by Tukey’s test (p < 0.05); uppercase letter for Ni levels and lowercase letters for seed levels. ** indicates that there was interaction between the factors according to least difference (Coty*Seed)
Elemental concentrations in treated plant tissues
The concentrations of Cu increased in soybean tissues following Ni application. In old leaves of plants which received solution Ni, there was a 65% increase in Cu. Although concentrations of Ca and Mg did not follow a distinct pattern, they tended to increase as Ni concentrations increased (Table 2). Plants grown from Ni-sufficient seeds had higher Mn concentrations in treatment that did not receive external Ni supply compared to those that did receive Ni (0.85 μM). However, for all tissues of plants grown from Ni-sufficient seeds, the average Mn concentration was 10.3% lower compared to the plants from Ni-depleted seeds. For Mn concentration, there were no statistically significant differences for the presence/absence of cotyledons in all tissues, except for the old leaves, regardless of the type of seeds used. Young leaves in plants that received Ni and retained the cotyledons had higher Mn concentrations relative to plants without cotyledons. Manganese concentrations in the stems of plants that did not receive Ni application were higher in plants with the cotyledons, relative to plants where the cotyledons were removed. In contrast, roots had higher Mn concentration in plants that had the cotyledons removed regardless of the Ni application. In general, soybean plants without Ni application and from Ni depleted seeds had higher K concentration compared with plants that received Ni application. Phosphorus concentrations were only statistically different in the stem tissues, whereas plants treated with Ni and without cotyledons had higher P concentrations. Finally, Zn concentrations were higher in plants that did not receive Ni, compared to plants treated with Ni.
Discussion
Trichomes: Pattern of nickel accumulation in leaf tissues after foliar nickel application
The cuticle plays a crucial role in protecting the plant against water loss through transpiration. Additionally, it serves to prevent the loss of organic and inorganic solutes from leaves due to rain. The cuticle contributes significantly to temperature regulation and plays a defensive role against pathogens and diseases (Kerstiens 1996; Riederer and Schreiber 2001; Marschner 2012). However, there are still gaps in our understanding as to how foliar-applied nutrients move across the leaf surface, including the potential role of trichomes (Fernández et al. 2017; Li et al. 2018, 2019). Using synchrotron-based μXRF in fresh leaves of soybean after foliar Ni application with two concentrations, we observed that Ni was concentrated in the pedicles of trichomes, while Mn concentrated in the trichome bases. Manganese localisation in trichomes base has previously been reported for sunflower (Blamey et al. 1986), pumpkin (Iwasaki and Matsumura 1999) and cucumber (Horiguchi 1987). The most likely reason for this is that during the absorption process by the root and possibly during transport to the shoot, Mn and Ni could compete for the same transport sites at the cell membranes (Demidchik et al. 2007). Divalent cations Ca, Mg, Fe, Cu, Ni, Mn, and Zn share characteristics that enable them to compete with one another in tasks like soil binding, root absorption, translocation, and subsequent utilization in the plant system. Another study examining the interaction of Zn and Cd in rice (Oryza sativa L.) vs. spinach (Spinacia oleracea L.) showed that Ni in shoots of rice and shoots and roots of spinach was significantly reduced by the increase of solution Zn (Wang et al. 2022).
Foliar-applied nickel is distributed quickly thought the trichomes: Synchrotron analysis
Synchrotron-based μXRF analysis of foliar applied Ni revealed that Ni moved quickly through the leaf surface, and was observed in the pedicle of trichomes 15 min. after foliar application (Figs. 2, 3, 4, 5 and 6). Thus, the results showed that trichomes are a primary pathway for the foliar uptake of Ni in soybean. Indeed, Ni appeared to have penetrated through the trichome underneath the Ni droplet, because after the exposure of Ni in soybean leaf there was a pathway following trichomes through the surrounding cells (Figs. 2, 3, 4 and 5). Nickel accumulated first within the trichomes, subsequently moved to the leaf cells, and depending on the time after exposure started, the concentration of Ni increased in the interveinal tissues. It has been reported that sunflower non-glandular trichomes (NGTs) are important for foliar Zn absorption (Li et al. 2019), but trichomes are not part of the primary pathway of foliar-applied Zn uptake in soybean and tomato (Li et al. 2018). Ohrui et al. (2007) showed that specialized trichomes can spread water quickly across the leaf surface by capillary effect, favouring the entire leaf tissue for the absorption of the water. Thus, it is also possible that soybean has a specialized mechanism for the absorption applied nutrient via leaf trichomes, as has already been noted for other plant species during environmental conditions stresses, such as drought (Winkler and Zots 2010; Vitarelli et al. 2016). The processes whereby foliar-applied nickel moves across the leaf surface and is translocated throughout the plant are not well understood (Bickford 2016), and major knowledge gaps in the physiological function of trichomes in taking up metal ions from foliar dosing remain.
Absorption of nickel and iron in soybean plants after nickel fertilization
Our study demonstrates for the first time that foliar-applied Ni (from NiSO4 solution) can be directly absorbed by trichome pedicles before then moving from underneath the Ni droplet toward the epidermal cells and other plant tissues through the veins. When Ni was supplied in the nutrient solution, the Ni was taken up by the roots before moving into the aboveground tissues and shoots (Table 2). The roots in direct contact with the nutrient solution had the highest Ni concentrations compared to other tissues, being 59.3% higher on average than the leaves. It was observed that more Fe was absorbed by the roots following Ni application. In a similar manner, Rahman et al. (2005) found that the supply of 10 μM Ni increased Fe concentration in roots in barley (Rahman et al. 2005). The Fe concentration can be significantly increased by the presence of Ni in the nutrient solution (Brune and Dietz 1995; Romheld 1991) given that Ni displaces Fe from FeDTPA and causes precipitation of Fe(OH)3 (Chaney 1988). A similar result has been reported by Freitas et al. (2019) in soybean, which demonstrated that Ni-supplied plants had increased Fe in the roots following Ni application. In a similar manner, in the study of Tiffin et al. (1973) which investigated the translocation of Fe from cotyledons, it was observed that the seed coats had significantly higher Fe concentrations, being ca. five-fold higher than the embryos. Furthermore, during the germination process, the radicles contained only 5% of the initial Fe content originally present in the seeds. A decreased concentration of Fe in plant leaves has been reported, which is analogous with the findings in the present study in highly Ni-responsive soybean (Piccini and Malavolta 1992). This phenomenon may be due to the ability of Ni to suppress specific root-to-shoot Fe signalling, which results in a reduction of transport of Fe to leaf tissues. Therefore, in this study, we supplied double the usual Fe concentration in solution to avoid inducing Fe deficiency. However, it appears that the supply of Ni had a negative effect on the development of soybean plants, with dry weight being lower in plants that received Ni fertilization. These results potentially indicate that the highest Ni concentrations can lead to Ni toxicity in the development of the soybean plants, such as interveinal chlorosis, without visual symptoms, compared to plants with normal Ni concentrations (Nagajyoti et al. 2010; Prasad et al. 2005; Reis et al. 2017).
Nickel fertilization and cotyledon absence or presence modulate urease activity
In plants grown from Ni-sufficient seeds which also received Ni treatment, it was observed that the urease activity increased in plants which had elevated Ni concentrations in the tissues that are involved in N storage and transport (Gerendás 1998; Witte 2011). Our findings that the urease activity is higher at elevated Ni concentrations agree with Freitas et al. (2018) and Barcelos et al. (2017, 2018), with these previous studies showing that leaf urease activity is responsive to Ni fertilization. The development of soybean plants was significantly enhanced by the presence of Ni from cotyledons, with the cotyledons being an important storage of Ni (and all nutrients) for soybean.
Seedling development after Ni application and presence of cotyledons
Urease activity was correlated with Ni concentration, as expected, in plants grown from Ni sufficient seeds and with Ni-dosed plants. Plants grown from Ni-sufficient seeds which received 0.85 μM L−1 Ni in solution, had a smaller shoot biomass relative to the treatment that did not receive Ni (Fig. 6). In contrast, in the treatment of Ni depleted seeds, the plants which received Ni and did not have the cotyledons removed produced higher biomass relative to the treatment in which the cotyledons were removed and did not receive Ni. Therefore, the Ni level within the cotyledons significantly influences both seedling growth and overall plant development.
Conclusions
Understanding foliar fertilization for enhancing crop micro-nutrition status holds importance. Nonetheless, the mechanism underlying the absorption of micro-nutrients by the leaves remains unclear. The Ni foliar application examined using synchrotron-based μXRF showed that the Ni-absorption occurred mainly by trichomes, specifically via the pedicle, showing that trichomes may be the primary pathway by which foliar applied Ni moves from the trichome pedicles toward the leaf surface and then to other plant tissues. In addition, urease activity, in which Ni plays a central role, was found to be correlated with Ni nutritional tissues status in plants grown from Ni sufficient seeds and with Ni-dosed plants. Even a low Ni seed concentration (<0.35 μg g−1 Ni) was enough to raise urease activity compared to the control. The presence of cotyledons resulted in greater biomass for plants that did not receive Ni application, emphasizing the importance of Ni as an essential nutrient for soybean growth but also the importance of cotyledons as nutrient stores. Nevertheless, elevated concentrations of Ni can lead to a reduction in biomass. Thus, determining the optimal Ni dosage is of paramount significance and further studies are required in this regard. This study deepens our understanding of the ability of plants to accumulate and translocate Ni into plant tissues, especially in well-nourished plants. Given that the leaf surface is covered with a cuticle and cuticular waxes, the underlying process whereby foliar-applied Ni is absorbed through the leaf surface by trichomes, via the pedicles, could contribute to a better understanding of micro-nutrient foliar absorption mechanisms, allowing the development of fertilizers with specific physical-chemical properties that favour greater absorption efficiency and micro-nutrient use by plants.
References
Bai C, Reilly CC, Wood BW (2006) Nickel deficiency disrupts metabolism of ureides, amino acids, and organic acids of young pecan foliage. Plant Physiol 140:433–443. https://doi.org/10.1104/pp.105.072983
Barcelos JPQ, Osório CRWS, Leal AJF, Alves CZ, Santos EF, Reis HPG, dos Reis AR (2017) Effects of foliar nickel (Ni) application on mineral nutrition status, urease activity and physiological quality of soybean seeds. Aust J Crop Sci 11:184–192. https://doi.org/10.21475/ajcs.17.11.02.p240
Barcelos JPQ, Reis HPG, Godoy CV, Gratão PL, Furlani Junior E, Putti FF, Campos M, Reis AR (2018) Impact of foliar nickel application on urease activity, antioxidant metabolism and control of powdery mildew (Microsphaera diffusa) in soybean plants. Plant Pathol 67(7):1502–1513. https://doi.org/10.1111/ppa.12871
Bates DM, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models usinglme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bickford CP (2016) Ecophysiology of leaf trichomes. Biol Commons 43:807–814. https://doi.org/10.1071/fp16095
Blamey FPC, Joyce DC, Edwards DG, Asher CJ (1986) Role of trichomes in sunflower tolerance to manganese toxicity. Plant Soil 91:171–180. https://doi.org/10.1007/bf02181785
Blamey AFPC, Paterson DJ, Walsh A, Afshar N, Mckenna BA, Cheng M, Horst WJ, Menzies NW, Kopittke PM (2018) Time-resolved X-ray fluorescence analysis of element distribution and concentration in living plants: an example using manganese toxicity in cowpea leaves. Environ Exp Bot 156:151–160. https://doi.org/10.1016/j.envexpbot.2018.09.002
Brown PH, Welch RM, Cary EE (1987) Nickel: a micronutrient essential for higher plants. Plant Physiol 85:801–803. https://doi.org/10.1104/pp.85.3.801
Brown PH, Welch RM, Madison JT (1990) Effect of nickel deficiency on soluble anion, amino acid, and nitrogen levels in barley. Plant Soil 125:19–27. https://doi.org/10.1007/bf00010740
Brune A, Dietz KJ (1995) A comparative analysis of element composition of roots and leaves of barley seedlings grown in the presence of toxic cadmium, molybdenum, nickel, and zinc concentrations. J Plant Nutr 18:853–868. https://doi.org/10.1080/01904169509364943
Chaney RL (1988) Plants can utilize iron form Fe‐N,N'‐di‐(2‐hydroxybenzoyl)‐ethylenediamine‐N,N'‐diacetic acid, a ferric chelate with 106 greater formation constant than Fe‐EDDHA. J Plant Nutr 11:6–11. https://doi.org/10.1080/01904168809363867
Ciurli S (2001) Electronic structure of the nickel ions in the active site of urease. Chemistry (Easton) 2001:99–100
Dabkowska-Naskret H, Jaworska H, Długosz J (2014) Assessment of the total nickel content and its available forms in the soils around cement plant Lafarge Poland. Int J Environ Res 8:231–236. https://doi.org/10.22059/ijer.2014.712
Dalton DA, Evans HJ, Hanus FJ (1985) Stimulation by nickel of soil microbial urease activity and urease and hydrogenase activities in soybeans grown in a low-nickel soil nickel has traditionally been considered of no major biological. Plant Soil 88:245–258. https://doi.org/10.1007/bf02182451
De Carvalho Braga Levy C, Mellis EV, Murrer MK, Inglés CR, Daynes CN, Cavalli E, Chiba MK (2019) Effects of nickel fertilization on soybean growth in tropical soils. Bragantia 78(3):432–443. https://doi.org/10.1590/1678-4499.20180242
Demidchik V, Shabala SN, Davies JM, Street D (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49:377–386. https://doi.org/10.1111/j.1365-313x.2006.02971.x
Dixon NE, Gazzola C, Blakeley RL, Zerner B (1975) Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 97:4131–4133. https://doi.org/10.1021/ja00847a045
Doolette CL, Read TL, Li C, Scheckel KG, Donner E, Kopittke PM, Schjoerring JK, Lombi E (2018) Foliar application of zinc sulphate and zinc EDTA to wheat leaves: differences in mobility, distribution, and speciation. 69: 4469–4481. https://doi.org/10.1093/jxb/ery236
Eskew DL, Welch RM, Cary EE (1983) Nickel: an essential micronutrient for legumes and possibly all higher plants. Scienc 222:621–623. https://doi.org/10.1126/science.222.4624.621
Eskew DL, Welch RM, Norvell WA (1984) Nickel in higher plants: further evidence for an essential role. Plant Physiol 76:691–693. https://doi.org/10.1104/pp.76.3.691
FAO (2017) FAOSTAT. Food and agriculture Organization of the United Nations. Rome, Italy
Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci 11:929–931. https://doi.org/10.2135/cropsci1971.0011183x001100060051x
Fernández V, Sotiropoulos T, Brown P (2013) Foliar fertilization: scientific principles and field pratices. International Fertilizer Industry Association (IFA)
Fernández V, Bahamonde HA, Peguero-pina JJ, Gil-pelegrín E, Sancho-knapik D, Gil L, Goldbach HE, Eichert T (2017) Physico-chemical properties of plant cuticles and their functional and ecological significance. J Exp Bot 68:5293–5306. https://doi.org/10.1093/jxb/erx302
Fernández V, Gil-Pelegrín E, Eichert T (2021) Foliar water and solute absorption: an update. Plant J 105:870–883. https://doi.org/10.1111/tpj.15090
Freitas DS, Rodak BW, Reis AR, Reis FB, Carvalho TS, Schulze J, Carneiro MAC, Guilherme LRG (2018) Hidden nickel deficiency? Nickel fertilization via soil improves nitrogen metabolism and grain yield in soybean genotypes. Front Plant Sci 9:1–16. https://doi.org/10.3389/fpls.2018.00614
Freitas DS, Rodak BW, Carneiro MAC, Guilherme LRG (2019) How does Ni fertilization affect a responsive soybean genotype? A dose study. Plant Soil 441:567–586. https://doi.org/10.1007/s11104-019-04146-2
Freitas MN, Guerra MBB, Adame A, Moraes TF, Lavres-Junior J, Pérez CA, Abdala DB, Cicero SM (2020) A first glance at the micro-ZnO coating of maize (Zea mays L.) seeds: a study of the elemental spatial distribution and Zn speciation analysis. J Anal At Spectrom 35:3021–3031. https://doi.org/10.1039/d0ja00282h
Gerendás J (1998) Influence of N and Ni supply on nitrogen metabolism and urease activity in rice (Oryza sativa L.). J Exp Bot 49:1545–1554. https://doi.org/10.1093/jxb/49.326.1545
Hogan ME, Swift IE, Done J (1983) Urease assay and ammonia release from leaf tissues. Phytochemistry 22:663–667. https://doi.org/10.1016/s0031-9422(00)86958-7
Horiguchi T (1987) Mechanism of manganese toxicity and tolerance of plants. Soil Sci Plant Nutr 33:595–606. https://doi.org/10.1080/00380768.1988.10415580
Hosseini H, Khoshgoftarmanesh AH (2013) The effect of foliar application of nickel in the mineral form and urea-Ni complex on fresh weight and nitrogen metabolism of lettuce. Sci Hortic 164:178–182. https://doi.org/10.1016/j.scienta.2013.09.030
Howard DL, de Jonge MD, Afshar N, Ryan CG, Kirkham R, Reinhardt J, Kewish CM, McKinlay J, Walsh A, Divitcos J, Basten N, Adamson L, Fiala T, Sammut L, Paterson DJ (2020) The XFM beamline at the Australian Synchrotron. J Synchrotron Rad 27:1447–1458. https://doi.org/10.1107/S1600577520010152
Iwasaki K, Matsumura A (1999) Effect of silicon on alleviation of manganese toxicity in pumpkin (Cucurbita moschata Duch cv. Shintosa). Soil Sci Plant Nutr 45:909–920. https://doi.org/10.1080/00380768.1999.10414340
Jaworska H, Bartkowiak A, Rózañski S (2013) The influence of anthropogenically increased pH on the content and the mobility of nickel in arable soils in the surroundings of Małogoszcz cement plant. Soil Sci Annu 64:14–18. https://doi.org/10.2478/ssa-2013-0003
Jones MWM, Kopittke PM, Casey L, Reinhardt J, Blamey FPC, van der Ent A (2020) Assessing radiation dose limits for X-ray fluorescence microscopy analysis of plant specimens. Ann Bot 125:599–610. https://doi.org/10.1093/aob/mcz195
Kerstiens G (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47(12):1813–1832. https://doi.org/10.1093/jxb/47.12.1813
Kopittke PM, Punshon T, Paterson DJ, Tappero RV, Wang P, Blamey FPC, van der Ent A, Lombi E (2018) Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiol 178:507–523. https://doi.org/10.1104/pp.18.00759
Krogmeier MJ, McCarty GW, Shogren DR, Bremner JM (1991) Effect of nickel deficiency in soybeans on the phytotoxicity of foliar-applied urea. Plant Soil 135:283–286. https://doi.org/10.1007/bf00010917
Kutman BY, Kutman UB, Cakmak I (2013) Foliar nickel application alleviates detrimental effects of glyphosate drift on yield and seed quality of wheat. J Agric Food Chem 61:8364–8372. https://doi.org/10.1021/jf402194v
Kutman BY, Kutman UB, Cakmak I (2014) Effects of seed nickel reserves or externally supplied nickel on the growth, nitrogen metabolites and nitrogen use efficiency of urea- or nitrate-fed soybean. Plant Soil 376:261–276. https://doi.org/10.1007/s11104-013-1983-7
Lavres J, Franco GC, Câmara GMS (2016) Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front Environ Sci 4:37. https://doi.org/10.3389/fenvs.2016.00037
Li C, Wang P, Menzies NW, Lombi E, Kopittke PM (2017) Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Ann Bot 120:405–415. https://doi.org/10.1093/aob/mcx063
Li C, Wang P, Lombi E, Cheng M, Tang C, Howard DL, Menzies NW, Kopittke PM (2018) Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J Exp Bot 69:2717–2729. https://doi.org/10.1093/jxb/ery085
Li C, Wang P, van der Ent A, Cheng M, Jiang H, Read TL, Lombi E, Tang C, De Jonge MD, Menzies NW et al (2019) Absorption of foliar-applied zn in sunflower (Helianthus annuus): importance of the cuticle, stomata and trichomes. Ann Bot 123:57–68. https://doi.org/10.1093/aob/mcy135
Licht OAB, Xuejing X, Qin Z, Miyazawa M, Ferreira FJF, Plawiak RAB (2006) Average reference values of geochemical and geophysical variables in stream sediments and soils, state of Paraná, Brazil. Bol Parana Geosci 59–87. https://doi.org/10.5380/geo.v58i0.10714
Lombi E, De Jonge MD, Donner E, Kopittke PM, Howard DL, Kirkham R, Ryan CG, Paterson D (2011) Fast X-Ray fluorescence microtomography of hydrated biological samples. PLoS One 6:e20626. https://doi.org/10.1371/journal.pone.0020626
Macedo FG, Bresolin JD, Santos EF, Furlan F, Silva WTL, Polacco JC, Lavres J (2016) Nickel availability in soil as influenced by liming and its role in soybean nitrogen metabolism. Front Plant Sci 7:1358. https://doi.org/10.3389/fpls.2016.01358
Macedo FG, Santos EF, Lavres J (2020) Agricultural crop influences availability of nickel in the rhizosphere; a study on base cation saturations, Ni dosages and crop succession. Rhizosphere 13:100182. https://doi.org/10.1016/j.rhisph.2019.100182
Marschner H (2012) Marschner’s mineral nutrition of higher plants. Elsevier, London
Morrison JM, Goldhaber MB, Lee L, Holloway JAM, Wanty RB, Wolf RE, Ranville JF (2009) A regional-scale study of chromium and nickel in soils of northern California, USA. Appl Geochem 24:1500–1511. https://doi.org/10.1016/j.apgeochem.2009.04.027
Nagajyoti PC, Lee KD, Tvm S (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Ohrui T, Nobira H, Sakata Y et al (2007) Foliar trichome- and aquaporin- aided water uptake in a drought-resistant epiphyte Tillandsia ionantha Planchon. Planta 227:47–56. https://doi.org/10.1007/s00425-007-0593-0
Ohyama T, Tewari K, Ishikawa S, Tanaka K, Kamiyama S, Ono Y, Hatano S, Norikuni O, Sueyoshi K, Hasegawa H et al (2017) Role of nitrogen on growth and seed yield of soybean and a new fertilization technique to promote role of nitrogen on growth and seed yield. In: In: Kasai M (ed) Soybean – the Basis of Yield, Biomass and Product. IntechOpen. https://doi.org/10.5772/66743
Ojeda-Barrios DL, Sánchez-Chávez E, Sida-Arreola JP, Valdez-Cepeda R, Balandran-Valladares M (2016) The impact of foliar nickel fertilization on urease activity in pecan trees. J Soil Sci Plant Nutr 16:237–247. https://doi.org/10.4067/s0718-95162016005000019
Oliveira JB, Lavres J, van der Ent A (2021) In situ analysis of nickel uptake from foliar application in pecan using instrumental μXRF analysis. J Soil Sci Plant Nutr 22:1–9. https://doi.org/10.1007/s42729-021-00599-6
Oliveira JB, Marques JPR, Rodak BW, Galindo FS, Carr NF, Almeida E, Araki K, Gonçalves JM, Reis AR, van der Ent A, Carvalho HWP, Lavres J (2022) Fate of nickel in soybean seeds dressed with different forms of nickel. Rhizosphere 21:100464. https://doi.org/10.1016/j.rhisph.2021.100464
Piccini DF, Malavolta E (1992) Effect of nickel on two common bean cultivars. J Plant Nutr:37–41. https://doi.org/10.1080/01904169209364478
Polacco JC, Mazzafera P, Tezotto T (2013) Opinion - nickel and urease in plants: still many knowledge gaps. Plant Sci 199–200:79–90. https://doi.org/10.1016/j.plantsci.2012.10.010
Prasad SM, Dwivedi R, Zeeshan M (2005) Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 43:177–185. https://doi.org/10.1007/s11099-005-0031-0
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404. https://doi.org/10.1081/pln-200049149
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Reis ARD, De Queiroz Barcelos JP, De Souza Osório CRW, Santos E, Lisboa LAM, Santini JMK, Santos MJDD, Furlani E, Campos M, De Figueiredo PAM, Lavres J, Gratão PL (2017) A glimpse into the physiological, biochemical and nutritional status of soybean plants under Ni-stress conditions. Environ Exp Bot 144:76–87. https://doi.org/10.1016/j.envexpbot.2017.10.006
Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52:2023–2032. https://doi.org/10.1093/jexbot/52.363.2023
Roca N, Pazos MS, Bech J (2008) The relationship between WRB soil units and heavy metals content in soils of Catamarca (Argentina). J Geochem Explor 96:77–85. https://doi.org/10.1016/j.gexplo.2007.04.004
Rodak BW, de Moraes MF, Pascoalino JAL, De Junior AO, de Castro C, Pauletti V (2015) Methods to quantify nickel in soils and plant tissues. Rev Bras Cienc do Solo 39:788–793. https://doi.org/10.1590/01000683rbcs20140542
Rodak BW, Freitas DS, Bernardes LF, Lima GJEO, Reis AR, Lavres J, Guilherme LRG (2021) Short-term nickel residual effect in field-grown soybeans: nickel-enriched soil acidity amendments promote plant growth and safe soil nickel levels. Arch Agron Soil Sci 68:1586–1600. https://doi.org/10.1080/03650340.2021.1912325
Romheld V (1991) The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: an ecological approach. Iron Nutr Interact Plants 130:127–134. https://doi.org/10.1007/bf00011867
Ruter JM (2005) Effect of nickel applications for control of mouse ear disorder on river birch. J Env Hort 23(1):17–20. https://doi.org/10.24266/0738-2898-23.1.17
Ryan CG (2000) Quantitative trace element imaging using PIXE and the nuclear microprobe. Int J Imag Syst Technol 11:219–230. https://doi.org/10.1002/ima.1007
Ryan CG, Jamieson DN (1993) Dynamic analysis: on-line quantitative PIXE microanalysis and its use in overlap-resolved elemental mapping. Nucl Instrum Methods Phys Res 77:203–214. https://doi.org/10.1016/0168-583x(93)95545-g
Ryan CG, Cousens DR, Sie SH, Griffin WL (1990) Quantitative analysis of PIXE spectra in geoscience applications. Nucl Instrum Methods Phys Res 49:271–276. https://doi.org/10.1016/0168-583x(88)90063-8
Ryan CG, Jamieson DN, Churms CL (1995) A new method for on-line true-elemental imaging using PIXE and the proton microprobe. Nucl Instrum Methods Phys Res 104:157–165. https://doi.org/10.1016/0168-583x(95)00404-1
Schreel JDM, Leroux O, Goossens W, Brodersen C, Rubinstein A, Steppe K (2020) Identifying the pathways for foliar water uptake in beech (Fagus sylvatica L.): a major role for trichomes. Plant J 103:769–780. https://doi.org/10.1111/tpj.14770
Scheckel KG, Lombi E, Rock SA, McLaughlin MJ (2004) In vivo synchrotron study of thallium speciation and compartmentation in Iberis intermedia. Environ Sci Technol 38:5095–5100. https://doi.org/10.1021/es049569g
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214. https://doi.org/10.1007/s11104-009-0193-9
Shimada N, Ando T (1980) Role of nickel in plant nutrition. II. Effect of nickel on the assimilation of urea by plants. Jpn J Soil Sci Plant Nutr 51:493–496
Tiffin LO, Chaney RL, Ambler JE (1973) Translocation of iron from soybean cotyledons. Plant Physiol 52:393–396. https://doi.org/10.1104/pp.52.5.393
Todd CD, Tipton PA, Blevins DG, Piedras P, Pineda M, Polacco JC (2006) Update on ureide degradation in legumes. J Exp Bot 57:5–12. https://doi.org/10.1093/jxb/erj013
van der Ent A, Przybyłowicz WJ, de Jonge MD, Harris HH, Ryan CG, Tylko G, Paterson DJ, Barnabas AD, Kopittke PM, Mesjasz-Przybyłowicz J (2018) X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phytol 218:432–452. https://doi.org/10.1111/nph.14810
van der Ent A, Echevarria G, Pollard AJ, Erskine PD (2019) X-ray fluorescence ionomics of herbarium collections. Sci Rep 9:4–8. https://doi.org/10.1038/s41598-019-40050-6
Vitarelli NC, Riina R, Cassino MF, Meira RMSA (2016) Trichome-like emergences in Croton of Brazilian highland rock outcrops: Evidences for atmospheric water uptake. Perspect Plant Ecol Evol Syst 22:23–35. https://doi.org/10.1016/j.ppees.2016.07.002
Wang M, Ma W, Chaney RL, Green CE, Chen W (2022) Comparative study on changes in Cd accumulation and ionome between rice and spinach: impact of zinc ion activity. J Environ Qual 5:26–34. https://doi.org/10.1002/jeq2.20418
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. Retrieved from https://ggplot2.tidyverse.org. Accessed 27 Dec 2023
Wickham H, François R, Henry L, Müller K (2022) dplyr: a grammar of data manipulation. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr. Accessed 27 Dec 2023
Wickham H, Vaughan D, Girlich M (2023) tidyr: tidy messy data. R package version 1.3.0. https://github.com/tidyverse/tidyr. Accessed 27 Dec 2023
Winkler U, Zotz G (2010) ‘And then there were three’: highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Ann Bot 106:421–427. https://doi.org/10.1093/aob/mcq120
Witte CP (2011) Urea metabolism in plants. Plant Sci 180:431–438. https://doi.org/10.1016/j.plantsci.2010.11.010
Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, Bonnin E (1983) Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn, and wheat. J Agric Food Chem 31:1240–1244. https://doi.org/10.1021/jf00120a024
Wood BW, Reilly CC, Nyczepir AP (2006) Field deficiency of nickel in trees: symptoms and causes. Acta Hortic 721:83–97. https://doi.org/10.17660/actahortic.2006.721.10
Acknowledgements
This research was undertaken on the XFM beamline at the Australian Synchrotron, part of ANSTO. We acknowledge the support of the AMMRF at the Centre for Microscopy and Microanalysis at The University of Queensland. We thank Amelia Corzo Remigio for assistance with the SEM analysis. We are grateful to Sao Paulo Research Foundation (FAPESP; 2019/04585-0) for the scholarship granted to Jessica Bezerra de Oliveira. JL thanks the “National Council for Scientific and Technological Development” of Brazil (“Conselho Nacional de Desenvolvimento Científico e Tecnológico” – CNPq) for the research fellowship (Grant number 303718/2020-0). We thank Pax Blamey for gifting the soybean seeds.
Author information
Authors and Affiliations
Contributions
JBO, JL and AVDE conceived the experiments. RLC advised on the design of the experiments. JBO undertook the hydroponics experiments and chemical analyses under supervision of AVDE. JBO, AVDE, HHH and PDE performed the synchrotron experiments with support from DLH. PMK and ARR helped to interpret the results and contributed to the writing. The manuscript was written by JBO and AVDE with input from all authors.
Corresponding author
Additional information
Responsible Editor: Adamo Domenico Rombolà.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1067 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, J.B., Lavres, J., Kopittke, P.M. et al. Unravelling the fate of foliar-applied nickel in soybean: a comprehensive investigation. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06567-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06567-0