Abstract
Background and aims
Cover cropping is a strategy to increase soil phosphorus (P) use efficiency in agroecosystems. We investigated adaptations on P acquisition strategies of nine cover crops grown in a calcareous and a non-calcareous chernozem with low available P.
Methods
Through a 108-day pot experiment using a calcareous and a decalcified chernozem, we evaluated black oat (Avena strigosa Schreb.), white lupin (Lupinus albus L.), narrow-leaf lupin (Lupinus angustifolius L.), phacelia (Phacelia tanacetifolia Benth.), berseem clover (Trifolium alexandrinum L.), buckwheat (Fagopyrum esculentum Moench), linseed (Linum usitatissimum L.), ramtil (Guizotia abyssinica [Lf] Cass.) and white mustard (Sinapis alba L.) for their dry biomass production, tissue P concentration and uptake, and effects on soil pH, phosphatase activity, mycorrhiza infection rate and soil P fractions.
Results
Cover crops differed in several parameters between the two soils. Dry biomass varied from 3.3 (white lupin) to 41.6 g pot-1 (mustard). Tissue P concentrations ranged from 0.046% (mustard) to 0.24% (clover). Species affected pH of both soils, ranging from − 0.66 to + 0.24. Acid phosphatase activity was higher in the decalcified soil, while alkaline phosphatases were higher in the calcareous soil. Root mycorrhizal infection rates ranged from 0 to > 50%. Most plants explored soil labile P exclusively, with organic P mineralization being more relevant in the calcareous soil.
Conclusion
We confirm that cover crops favoured distinct strategies to access the predominant soil labile P forms in each soil. Mycorrhizal species were particularly efficient in the decalcified soil, while species with high phosphatase secretion accessed higher Po, especially in the calcareous soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhancing phosphorus (P) management in agriculture is a complex but crucial task to guarantee the stability of agri-food sectors worldwide. Uneven P distribution on the globe and uprising geopolitical tensions worldwide mean that efficient P use is now a more critical matter than ever before. Similar to most western countries, overfertilization in Austria has led to the accumulation of large soil total P (Pt) stocks (Zoboli et al. 2016). However, complex interactions such as fixation in Fe and Al (oxy)hydroxides and precipitation as Ca-P, can rapidly render 95–99% of the Pt unavailable for plant uptake, even in the long term (Asomaning 2020). The resulting low P availability can lead to deficiencies and limit crop productivity (Vance et al. 2003).
To tackle this challenge, cover cropping has emerged as a promising approach to make better use of limited P resources. Certain plant species have shown the ability to acquire P efficiently, which is essential for their growth. These species possess specific traits that aid in P acquisition. These traits include displaying high internal P-use efficiency (Hallama et al. 2019), symbiotic relationships with arbuscular mycorrhizal fungi (AMF, Plenchette et al. 2011), high potential activity of phosphatases (Maseko and Dakora 2019; Nannipieri et al. 2011), high rhizosphere acidification capacity (Oburger et al. 2011), adapted root structures, and promoting soil microbial activity (Schilling et al. 1998).
Cover cropping is a well-established practice; its use is often centred around soil coverage and erosion reduction, mitigation of nitrogen leaching, soil organic matter accumulation, and soil chemical, physical and biological improvement. Soil nutrient cycling is often stated as a benefit of cover cropping; however, it has seldom been characterised for nutrients other than nitrogen. Therefore, its effects on P pools and availability are overlooked in practice or are, at best, seen as a secondary benefit. Studies concerned with cover crops’ effects in soil P availability have been eloquently summarized by Hallama et al. (2019), who concluded that although P related benefits of cover crops can generally be observed, they vary highly between species and site characteristics. Therefore, these effects need to be investigated carefully in different environments, soils, crops and climatic conditions.
In this research, we investigate how both popular (lupines, white mustard, buckwheat, black oat) and less frequently used cover crop species (ramtil, linseed, phacelia and clover) adapt their P-acquisition strategies when grown in a decalcified and a calcareous chernozem. These species and soils were selected due to their importance in the Austrian region of Lower Austria (Bock 2014), the most important agricultural region in the country, representing ~44% of the countries’ crop value output in 2021 (REAA 2021).
We hypothesize that (1) cover crops differentiate their P-acquisition strategies to take up the predominant P forms in each soil, (2) rhizosphere acidification and phosphatase activity is more relevant for P acquisition in the calcareous soil, as it increases dissolution of easily soluble Ca-Pi,o forms, and (3) mycorrhization is more important in the decalcified soil due to the predominance of labile Pi forms.
In this study, we explore the interactions and differentiation of cover crop P-acquisition strategies and investigate how they facilitate the uptake of specific P fractions in soils with differential properties. These insights enable a better-informed selection of cover crop species tailored to the unique characteristics and predominant P-species of diverging soils, supporting researchers and farmers who are looking to improve P management and overall soil health.
Materials and methods
To assess the cover crop P acquisition strategies and their effects on the P fractions of two Austrian soils, a greenhouse pot experiment was conducted for 108 days. The soils were collected from the topsoil (0–20 cm) of two sites in Lower Austria and were selected for their low available P, relatively high Pt, contrasting pH, CaCO3 content, and texture. Both soils were classified according to the international soil classification system (IUSS Working Group 2014). The first, a clayey chernozem, was collected from an arable plot in Langenlebarn – AT, with a decalcified A horizon. The second, a calcareous loamy chernozem, was collected from an experimental site in Groß-Enzersdorf – AT.
Prior to establishment, both soils were analysed for pH (0.01 M CaCl2 ) at a 1:2.5 (soil:solution) ratio (pH meter inoLab®Multi 9620 IDS), total P (aqua regia digestion); Olsen-P (0.5M NaHCO3 pH 8.5) according to Olsen and Sommers (1982); maximum water holding capacity (MWHC) as described by ISO (2019) and CaCO3 via dissolution in excess 1M HCl followed by titration with a 1M NaOH solution (Horváth et al. 2005, Table 1). Total carbon and total organic carbon were obtained through combustion at 400 °C and at 600 °C, respectively using a soli TOC (Elementar Analysensysteme GmbH, Langenselbold, Germany).
Experimental setup and treatments
For each soil, the cover crops tested and their sowing density can be seen in Table 2. One control treatment with no plant was added for each soil, resulting in a (9 + 1) × 2 design with 4 replicates. Pots were filled with 2 kg of air-dried and sieved (1 cm) soil and placed inside the greenhouse in a randomized block design. Pots were moved twice a week to decrease positioning effects. Irrigation was supplied manually with tap water, and soils were maintained at 60-70% MWHC throughout the experiment. The temperature in the greenhouse varied between 16 °C (night) and 30 °C (day).
Establishment and conduction
Seeds were sown to a depth of 3 cm on Jan 25th 2022. Sowing density was chosen based on the regional field density for each crop, multiplied by three. Twenty days after sowing (DAS), plants were thinned to match the target number of plants per pot for each species. Weeds were removed manually.
Fertilization
To ensure P as the only growth limiting nutrient, no P was applied in any form, while other nutrients were supplemented in sufficient amounts through nutrient solutions. At 3 DAS, 230 mg kg−1 N, 310 mg kg−1 K, 45 mg kg−1 Mg, 65 mg kg−1 S, 107 mg kg−1 Ca were applied to every pot. At 35 DAS, Fe (3 mg kg−1), Zn (3 mg kg−1), Mn (3 mg kg−1), Cu (1.5 mg kg−1), B (0.3 mg kg−1) and Mo (0.3 mg kg−1) were also added. At 50 DAS, a second application of N (150 mg kg−1) and K (50 mg kg−1) was conducted. The composition of the nutrient solutions can be seen in Online Resource 1.
Sampling and analyses
The plants were harvested on May 13th 2022 (108 days cycle). At harvest, the whole shoot and root biomass of plants was collected and cleaned with tap water to remove dirt particles. Subsamples of roots were collected and stored at 4 ºC in 30% ethanol solution for arbuscular mycorrhiza infection rate (%AMF) determination. These subsamples were stained according to Vierheilig et al. (1998) and %AMF was determined through the Gridline intersect method (Giovanetti and Mosse 1980).
The plant shoots and remaining roots were oven-dried at 60 ºC for 48 h, weighed for dry biomass yield, milled to 2 mm and subjected to microwave digestion (HNO3 + H2O2). Shoot and root P concentrations were determined using ICP-OES (Optima 8300, PerkinElmer, Massachusetts, USA). By dividing the plant’s total P uptake (mg pot−1) by their root dry biomass (g pot−1), we calculated the root P acquisition efficiency (mg g−1) of the individual species in each soil, a parameter which represents the amount of P acquired per unit root biomass.
At harvest, rhizospheric soil samples were collected by gently shaking the soil attached to the roots into plastic bags and were then divided into two subsamples. One was air-dried, sieved at 2 mm and used for pH (CaCl2) determination and P fractionation according to Hedley et al. (1982) as modified by Rheinheimer (2000). The fractionation allows the extraction of inorganic (Pi) and organic (Po) P forms, which were grouped in labile (anion exchange resin membrane extracted Pi [PResin] and 0.5M NaHCO3 extracted Pi,o); moderately labile (0.1M NaOH extracted Pi,o and 1M HCl extracted Pi); and non-labile P pools (0.5M NaOH extracted Pi,o and residual P). The P in the extracts was determined via the phosphomolybdate blue method (Murphy and Riley 1962).
A second soil subsample was stored at ambient humidity at 4 ºC, and was used for the determination of the potential activity of the acid (APASE) and alkaline (AlkPASE) phosphatases, obtained through colorimetric determination of the p-nitrophenol released per kg of soil (corrected for humidity) per hour (mg kg−1 h−1) after incubation at 37 ºC with a p-nitrophenyl phosphate (Sigma N4645) buffered solution (pH 6.5 and 11.0, respectively), according to Tabatabai and Bremmer (1969).
Finally, all quantitative parameters analyzed were ranked in a scale of 0-100, where the highest grade (100) was conferred to the highest average value for each parameter considering only the species with observations in both soils (lupines and mustard excluded to determine the 100). All other replicates of treatments were graded in proportion to the highest values observed, and the average grades and standard deviations were displayed in a table, allowing a visual comparison of the cover crops and their performance in the soils.
Statistical analyses
All quantitative data were tested for homogeneity of variance and normality of the residuals by the Levene and Shapiro–Wilk tests, respectively. They were then subjected the two-way ANOVA analysis of variance to determine the effects of plant species and soil type and their interaction on the measured variables. Means were compared through Tukey’s HSD test, except the calculated parameter ‘root P acquisition efficiency’, which was subjected to a pairwise comparison through a t-test to determine differences between the same plants in each soil. The cut-off for statistical significance was taken as p ≤ 0.05 for all analyses. All tests were performed using RStudio version 4.2.2. All figures were drawn using a combination of Microsoft Excel and PowerPoint.
Results
Plant biomass and P parameters
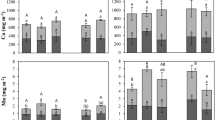
Shoot and root dry biomass (Fig. 1a, b), P concentration (Fig. 1c, d) and total P uptake (Fig. 1e, f) in both soils are presented for each cover crop species. All data for the narrow-leaf lupin in the the calcareous soil, however, was omitted due to the crop’s high sensitivity to soil pH ≥ 6.0 (Tang et al. 1993) and high Ca environments (Ding et al. 2018), which resulted in its premature senescence in this soil. All other crops had their results reported for completeness; however, due to impaired development of the narrow-leaf lupin and the mustard in the decalcified soil as well as the white lupin in the calcareous soil, these are not discussed in detail and are excluded from the summary comparison in the discussion. All other crop species were shown to develop well in a range of soil types (Myers and Meinke 1994, Ranganatha et al. 2013, Ugrenović et al. 2021, Casa et al. 1990, Kilian 2016).
Cover crops shoot and root dry mass (g pot−1), root and shoot P concentrations (g kg−1) and P uptake (mg pot−1) in the decalcified (a, c, e) and calcareous (b, d, f) soils. Means of the totals (n = 4) followed by the same letter did not differ significantly in the Tukey test at p ≤ 0.05. **=data not reported
In terms of biomass, the black oat and buckwheat performed comparatively well and consistently in both soils. The mustard under-performed in the decalcified but was the best performing cover crop in the calcareous soil. Conversely, the biomass of white lupin was statistically equal to buckwheat, linseed and ramtil in the decalcified soil, but was the lowest among all species in the calcareous soil. Overall, crops produced higher biomass in the latter except for the white lupin and the clover.
Plant shoot and root P concentrations across the different species were generally similar when they were grown in the same soil. However, higher concentrations were observed in crops grown in the calcareous soil, following a similar pattern of that of biomass. Clover and linseed had the highest P concentrations in both soils, indicating low internal physiological P-use efficiency (high P requirement for biomass production). Meanwhile, ramtil showed an above average P concentration, being higher than white lupin, phacelia and mustard in decalcified soil, and higher than buckwheat in the calcareous soil.
Due to these differences in P concentrations, total P uptake by the cover crops followed a slightly different pattern than that observed for biomass, with clover, linseed and ramtil showing comparable or higher P uptake than those obtained by plants with greater biomass. The clover P uptake was comparable to that of the black oat despite its lower biomass in the decalcified soil and comparable to that of phacelia in calcareous soil, despite its significantly lower biomass production in both soils. The P uptake of linseed and ramtil, in turn, was even higher in this soil, being similar to that of black oat regardless of their significantly lower biomass production.
The root P acquisition efficiency of the individual species in each soil is shown in Table 3. This parameter revealed a significantly higher root P acquisition efficiency for linseed and ramtil in the calcareous soil than in the decalcified soil.
Root activity and soil phosphorus fractions
Soil pH
Variations in rhizospheric soil pH (Δ pH) when compared to the controls before establishment are shown in Fig. 2. While soil pH did not vary in both control treatments when compared to the original soils, all species acidified the soil except for the mustard (both soils), and the white lupin and clover in the calcareous soil. Buckwheat acidified the soil more strongly than all other plants in the decalcified soil (-0.67 ± 0.10), and more strongly than white lupin in the calcareous. All plants except buckwheat acidified the soils on average by 0.51 ± 0.15 pH units, and their effects were not significantly different from each other.
Arbuscular mycorrhizal fungi (AMF) infection
Root colonization with AMF (Fig. 3) was observed for certain crops in both soils. AMF infection was not observed in lupines, buckwheat and mustard in either soil. In the decalcified soil, all crops known to form mycorrhizal symbiosis displayed comparable infection rates, with the only significant difference being between clover (56.7%) and ramtil (29.9%). In the calcareous soil, however, black oat showed a similar proportion of infected roots (58.4%) to that of linseed (57.1%), which were significantly higher than that of the clover, ramtil and phacelia, which was only colonized in this soil.
Potential phosphatase activity
The potential acid (APASE) and alkaline (AlkPASE) phosphatase activity varied significantly across treatments (Fig. 4). Overall, higher APASE levels were observed for control and all crops in the decalcified soil, while the AlkPASE was higher in the calcareous soil. In the former, phacelia, buckwheat and black oat promoted the highest APASE levels, while clover and ramtil also performed above the control baseline activity (140.6 ± 7.9), indicating plant effects. For AlkPASE, buckwheat promoted the highest values, followed by phacelia, black oat, ramtil and linseed, all of which performed above the control baseline (84.6 ± 6.3).
Potential acid and alkaline phosphatase activity (mg h−1 kg−1) as affected by cover crop species in the decalcified (a) and calcareous (b) soils. Means (n = 4) followed by the same letter in the same case did not differ significantly in the Tukey test at p ≤ 0.05. Error bars represent standard deviation. **=data not reported
In the calcareous soil, significantly higher levels of APASE compared to the control baseline (102.3 ± 6.4) were obtained by black oat, buckwheat, linseed, ramtil and mustard. Meanwhile, black oat, phacelia, linseed, ramtil and mustard, all increased AlkPASE at similar rates and were higher than control (232.7 ± 29.6). Although all crops had higher AlkPASE in this soil, the mustard can be highlighted here, since it did not promote any changes in this parameter in the decalcified soil. In the calcareous soil, however, it showed the highest AlkPASE activity, which may be related to its higher biomass production in this soil.
Soil P fractions
During the 108-day cycle, no variations in P fractions were observed for the controls compared to their values before establishment (Online resource 2). The plant × soil interaction affected soil PResin and PoNaHCO3 (labile fractions) significantly. No significant reductions in soil PiNaHCO3 or in any fraction of the moderately and the non-labile soil P pools were observed in either soil. The complete data on soil P fractions and pools can be accessed in Online resource 3. Data on labile P removal discriminated by extractant can be seen in Fig. 5. Strong linear correlations were found between labile P removed from the soil and the crop’s total P uptake, with R² = 0.79 in the decalcified soil and R² = 0.78 in the calcareous soil (Online Resource 4).
Soil labile P fractions (mg kg−1) removed by cover crop species in the decalcified and calcareous soils in comparison to the control treatments. Within individual P fractions, means (n = 4) followed by the same letter (in the same order as the bars are displayed) did not differ significantly in the Tukey test at p ≤ 0.05. Error bars represent standard deviation. **=data not reported
Overall, higher PResin and PoNaHCO3 removal was seen in the calcareous soil. In this soil, linseed, black oat, ramtil and mustard showed the highest PResin removal. In the decalcified soil, black oat took up the most PResin while the clover was consistent in the exploration of this fraction in both soils. Interestingly, despite significantly higher soil organic matter in the decalcified soil (4.7%) when compared to the calcareous (3.0%), the latter had significantly more PoNaHCO3 removed, which, associated with its higher values for this P pool, reveal a different Po content in each soil (the former with higher PoNaOH 0.1M).
The soil PoNaHCO3 reduction was strongly correlated with the AlkPASE in both soils (Fig. 6), including the exceptional values for both parameters seen in buckwheat in the decalcified soil.
Discussion
Cover crop P acquisition strategies and intensities are influenced by plant-soil interactions, varying substantially between environments, climates and production systems (Hallama et al. 2019). Here, we explore how nine cover crop species differentiate their P-acquisition strategies when grown on a decalcified and a calcareous chernozem. Specifically, changes in plant biomass production, tissue P concentration, total P uptake, AMF colonization rates, potential activity of APASE and AlkPASE, as well as soil ΔpH and P fractions are investigated.
In calcareous soils, CaCO3 dissolution increases the activity of Ca2+ and HCO3−. Depending on the solution Ca:P ratios (Wang and Nancollas 2008), these species contribute to the precipitation of Ca-P forms such as monocalcium (Ca(H2PO4)2), dicalcium (CaHPO4) and tricalcium phosphate (Ca3(PO4)2) thereby reducing P plant availability (Geng et al. 2022). Additionally, lime dissolution releases OH−, neutralizing direct (H+) and indirect (Al3+) sources of acidity (Hinsinger 1998; Havlin et al. 2017). Rhizosphere acidification, therefore, can increase P availability by solubilizing Ca-P (Baccari and Krouma 2023).
In our study, cover crops acidified both soils at similar rates, despite the higher buffering capacity of the calcareous soil, indicating that H+ release was an important P acquisition strategy for these plants in this soil. The slight alkalinizing effect (although statistically non-significant) by the mustard in both soils has also been reported for Sinapis alba L. (Schwerdtner and Spohn 2022) and Brassica juncea L., a species of same family (Kim et al. 2010). In our experiment, we hypothesize that the nitrogen form (NH4NO3) applied later in the cycle could have caused an imbalance in the cation-anion uptake by the mustard, minimizing its effects on soil pH. In the decalcified soil, the low biomass might not have been sufficient to promote significant acidification in the mustard rhizosphere, while in the calcareous soil the mustard could have tapped into the NO3− after quick NH4+ depletion due to its high biomass, neutralizing the acidity generated.
High biomass production is one of the most important traits for cover crops regardless of their P acquisition strategies (Aime et al. 2020). In our study, biomass contrasts between soils can be explained partially by the significantly higher available P values observed in the calcareous soil, both for Olsen-P and PResin. Although Olsen-P suggest a smaller difference in available P between soils (27% higher in the calcareous soil), the resin test pointed at a much higher difference of 71%. Possibly, the Olsen extraction (NaHCO3 at pH 8.5) in the calcareous soil might not have extracted certain P compounds (e.g. weakly bound monocalcium phosphates) that were released at the lower pH levels of the resin extraction. Differences in biomass can also be attributed to specific plant tolerance to Ca2+ (calcifuges vs. calcicoles, White and Broadley 2003), which affected the growth of both lupines in the calcareous soil.
Moreover, high P accumulation is also relevant because plant biomass production is tightly associated with genetic factors (Ain et al. 2022). Since the mustard has a high biomass production potential, its low P uptake and yield in the decalcified soil was likely due to the small volume of soil used and the soils’ native low P levels. In turn, the clover and the linseed naturally yield lower biomass, requiring less resources to reach their growth potential and may obtain high P uptake even under limiting conditions. The tissue P concentrations (dry weight basis) observed in our experiment ranged from 0.046% (mustard in the decalcified soil) to 0.24% (clover in the calcareous soil), which is within the lower half of the 0.05–0.5% range often reported in literature (Johri et al. 2015). This was expected since no P was applied to the already impoverished soils.
Interestingly, despite non-significant differences in the biomass of linseed and ramtil in each soil, the higher root P acquisition efficiency observed for these species in the calcareous soil allowed for significantly higher P uptake, indicating their adaptability to the calcareous soil. This higher efficiency was also likely associated with the higher AlkPASE activity observed for both species in the calcareous soil compared to the decalcified soil, which resulted in two of the highest Po removal rates among all cover crops in both soils. The clover had a high root P acquisition efficiency in both soils, showing certain adaptability to chemically and physically divergent soils.
Another factor contributing to crop P acquisition was the symbiosis between AMF and roots, which can increase the explored soil volume up to 40 times (Giovannetti et al. 2001). In our study, no AMF were observed in the roots of lupines, buckwheat and mustard, all of which have been reported to be weak or non-hosts (Lambers et al. 2013; Likar et al. 2008; Vierheiligh et al. 2003). Phacelia was the only species to show AMF infections in only one soil (calcareous), a species that has been reported to host some AMF strains (Cripps and Eddington 2018; Casanova-Katny et al. 2011), but also to not be infected in many soils (Bacq-Labreuil et al. 2019). Because no AMF were inoculated in our experiment, the plants depended on the soil’s native fungal communities to be infected. An interesting aspect is, that despite similar AMF infection rates in both soils, host species consistently took up higher Pi than non-hosts in the decalcified soil. As AMF can only directly absorb labile Pi forms (Antunes et al. 2007; Etesami et al. 2021), the increased soil volume exploration promoted by the AMF might have been more important in the decalcified soil due to its lower available P content.
AMF can also recruit and stimulate phosphatase-secreting microorganisms and can secrete phosphatases themselves (Della Mónica et al. 2018), which aided Po mineralization especially in the calcareous soil. In our experiment, the AMF hosts black oat, phacelia, linseed and ramtil, had the highest AlkPASE activities, which are exclusively secreted by microbes (Spohn and Kuzyakov 2013; Hallama et al. 2019). These results also corroborate with Peng et al. (2020), who reported higher AlkPASE under AMF inoculated alfalfa (Medicago sativa L.) when compared to their non-inoculated counterparts.
One exception to this rule was the mustard in the calcareous soil, which had high AlkPASE activity despite no AMF colonization, possibly because it did not acidify the soil. Slight soil alkalinity could have stimulated higher AlkPASE activity, since its optimal pH range is between 8.5 and 11, while for APASE it is between 4 and 6.5 (Niemi and Vepsäläinen 2005; Nannipieri et al. 2011). For this reason, AlkPASE activity was overall greater in the calcareous soil, while APASE levels were higher in the decalcified soil. In the latter, the non-AMF-colonized phacelia and buckwheat also stimulated high AlkPASE activities, which could indicate significant participation of their root exudates (e.g. organic acids, phenolic compounds, sugars and amino acids) in stimulating microbial activity (Kalinova et al. 2007; Hallama et al. 2021).
Black oat, phacelia and buckwheat had the highest APASE activities while also promoting the highest pH decreases in the decalcified soil, suggesting a synergy between both strategies. In the calcareous soil, only black oat and buckwheat maintained high APASE activity, while the AlkPASE activity of phacelia increased. This shows that while soil pH seems to have determined the baseline levels of phosphatase activities in each soil, black oat and buckwheat showed the capacity to still exude high APASE levels even in the calcareous soil, indicating the importance of this strategy to these species under both soil conditions.
The discussed differential P acquisition strategies resulted in the exploration of different soil P fractions. Despite the contrasts in the P-acquisition strategies of the investigated species, the moderately and non-labile P pools were virtually unexplored in a 108-day growth cycle in both soils. In a field experiment, Soltangheisi et al. (2020) reported that cover crops grown on non-P-fertilized soils did not significantly alter any soil P pools. In our experiment, however, most species successfully accessed labile P in both soils, which can be explained by the lower soil volume available for plants grown in pots and the ease of representative sampling when compared to field conditions.
In the calcareous soil, the higher share of PResin taken up are explained by its higher native available P content in comparison to the decalcified soil. A clear difference between soils, however, was the higher contribution of labile Po to total P uptake in the calcareous soil, represented by this soil’s significantly higher initial PoNaHCO3 levels and reduction rates through plant uptake. The high moderately labile organic P (Online Resource 3) observed in this soil can be explained by Ca2+ flocculation of organic matter (predominant in the calcareous soil), which promotes the formation of larger and more stable organic matter aggregates, reducing its solubility and protecting it from microbial degradation (Wuddivira and Camps-Roach 2006). With the depletion of the labile P pools, however, slow mineralization of this Po could be further made available by AlkPASE activity, which could play a crucial role in increasing P availability to plants (Nannipieri et al. 2011).
Interestingly, all crops showing significant PoNaHCO3 reduction also displayed comparatively high AlkPASE, especially seen for buckwheat in the decalcified soil, and for mustard, linseed, ramtil, black oat and phacelia in the calcareous soil. This is important due to crops inability to take up Po directly, requiring its mineralization to Pi forms prior to absorption (Hayes et al. 2000). In turn, this mineralized P buffers other P pools, such as the PiNaHCO3, which explains its seemingly low removal rates. It is likely that the removal of PiNaHCO3 by these species is replenished by Po mineralization, increasing Pi availability, but also leading to no statistical differences between the observed levels of the Pi fraction among soils or crops.
The P acquisition parameters analysed in this study were combined and transformed to a qualitative ranking for the comparison of cover crop species performance in each soil (Table 4).
This ranking shows that, except for the legume species, all cover crops performed better in biomass production, P concentration and P uptake in the calcareous soil. While this probably is related to its higher initial Pi levels, Po also played a more important role for P supply to plants in this soil. This is because the high initial pH of this soil allowed for generally higher alkaline phosphatase activity levels, which contributed to a greater Po mineralization when compared to the decalcified soil. In the latter, the overall higher levels of soil acidification and acid phosphatase activity obtained by cover crops can be explained by its lower initial pH and buffering capacity in comparison to the alkaline and calcareous soil. Interestingly, the buckwheat promoted high activity for both phosphatases in both soils, mineralizing comparatively high Po in the decalcified but taking up high Pi in the calcareous soil, showing a differential contribution of P pools to this plant species in each soil.
Conclusions
Cover crops adapted their P-acquisition strategies to the predominant soil P fractions in a calcareous and a decalcified chernozem. The species tested accessed mostly labile P forms (PResin, PNaHCO3). In the decalcified soil with lower labile P (predominantly Pi), the mycorrhizal species black oat, clover, linseed, and ramtil excelled in Pi uptake, likely attributable to their extensive soil exploration. In the calcareous soil, Po played a more important role in plant nutrition. In this soil, black oat, buckwheat, phacelia, linseed, ramtil, and mustard increased AlkPASE activity levels (also seen for buckwheat in the decalcified soil), which correlated positively with Po removal. Additionally, despite the high pH buffering capacity of the calcareous soil, black oat and buckwheat promoted particularly high rhizosphere acidification in this soil. This indicates an important participation of acidification to P acquisition for these species, which synergistically exhibited elevated activity of APASE in this soil. The activity of both phosphatases was particularly important in the calcareous soil, suggesting the hydrolysis of Po. This confirms that rhizosphere acidification and phosphatase activity was more relevant in the calcareous soil, and that mycorrhization was more important in the decalcified soil. Consequently, in P-deficient soils dominated by labile Pi, mycorrhizal species such as black oat, clover, linseed and ramtil may be more suitable for P exploration.
The high root P acquisition efficiency of linseed and ramtil in the calcareous soil suggests suitability to P acquisition in high Ca environments. Additionally, the clover and the linseed had the highest tissue P concentrations, which resulted in relatively high P uptakes despite low biomass, making these species strong candidates for P cycling in scenarios which limit biomass production potential. The lupines did not take up high amounts of P in either soil in our experiment, and thus are not recommended for P mining in these conditions. On the other hand, black oat and mustard can be very efficient for P acquisition, the latter already being extensively used in Lower Austria as cover crop. Our results show that the cover crops tested in differentiated their P acquisition strategies according to soil characteristics such as pH, Ca content and predominant P forms. As no single mechanism completely explained plant P concentrations and P uptake, the P acquisition efficiency of these cover crops is due to a combination of P mobilization strategies.
Data availability
The data supporting the findings of this study are available upon reasonable request.
References
Aime RS, Zehnder GW, Talley C, Narayanan S (2020) Differences in biomass production and water use efficiency among seven different cover crops in the wet winter seasons of 2016/17 and 2018 in South Carolina. Agronomy 10(4):463. https://doi.org/10.3390/agronomy10040463
Ain NU, Haider FU, Fatima M, Habiba, Zhou Y, Ming R (2022) Genetic determinants of Biomass in C4 crops: Molecular and Agronomic approaches to increase Biomass for Biofuels. Front Plant Sci 23(13):839588. https://doi.org/10.3389/fpls.2022.839588
Antunes PM, Schneider K, Hillis D, Klironomos JN (2007) Can the arbuscular mycorrhizal fungus Glomus intraradices actively mobilize P from rock phosphates? Pedobiologia 51:281–286. https://doi.org/10.1016/j.pedobi.2007.04.007
Asomaning SK (2020) Processes and factors affecting phosphorus sorption in soils. In: Kyzas G, Lazaridis N (eds) Sorption in 2020s. IntechOpen. https://doi.org/10.5772/intechopen.90719
Baccari B, Krouma A (2023) Rhizosphere acidification determines phosphorus availability in calcareous soil and influences faba bean (Vicia faba) tolerance to P Deficiency. Sustainability 15(7):6203. https://doi.org/10.3390/su15076203
Bacq-Labreuil A, Crawford J, Mooney SJ, Neal AL, Ritz K (2019) Cover crop species have contrasting influence upon soil structural genesis and microbial community phenotype. Sci Rep 9:7473. https://doi.org/10.1038/s41598-019-43937-6
Bock P (2014) Chernozems in the Austrian and WRB classification system. Available via https://abstracts.boku.ac.at/download.php?dataset_id=12877&property_id=107. Accessed 03 May 2023
Casa R, Russell G, Lo Cascio B, Rossini F (1990) Environmental effects on linseed (Linum usitatissimum L.) yield and growth of flax at different stand densities. Euro J Agronomy 11:3–4. https://doi.org/10.1016/S1161-0301(99)00037-4
Casanova-Katny MA, Torres-Mellado GA, Palfner G, Cavieres LA (2011) The best for the guest: high andean nurse cushions of Azorella Madreporica enhance arbuscular mycorrhizal status in associated plant species. Mycorrhiza 21:613–622. https://doi.org/10.1007/s00572-011-0367-1
Cripps CL, Eddington LH (2018) Distribution of mycorrhizal types among Alpine Vascular Plant families on the Beartooth Plateau, Rocky Mountains, USA, in reference to large-scale patterns in Arctic–Alpine habitats. Arct Antarct Alp Res 37:177–188. https://doi.org/10.1657/1523-0430(2005)037[0177:DOMTAA]2.0.CO;2
Della Mónica IF, Godoy MS, Godeas AM, Scervino JM (2018) Fungal extracellular phosphatases: their role in P cycling under different pH and P sources availability. J Appl Microbiol 124(1):155–165. https://doi.org/10.1111/jam.13620
Ding W, Clode PL, Clemens JC, Lambers H (2018) Sensitivity of different Lupinus species to calcium under a low phosphorus supply. Plant Cell Environ 41(7):1512–1523. https://doi.org/10.1111/pce.13179
Etesami H, Jeong BR, Glick BR (2021) Contribution of Arbuscular Mycorrhizal Fungi, phosphate-solubilizing Bacteria, and Silicon to P Uptake by Plant. Front Plant Sci 12:699618. https://doi.org/10.3389/fpls.2021.699618
Geng Y, Pan S, Zhang L, Qiu J, He K, Gao H, Li Z, Tian D (2022) Phosphorus biogeochemistry regulated by carbonates in soil. Env Res 214(2):113894. https://doi.org/10.1016/j.envres.2022.113894
Giovanetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84(3):489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Giovannetti M, Fortuna P, Citernesi AS, Morini S, Nuti MP (2001) The occurrence of anastomosis formation and nuclear exchange in intact arbuscular mycorrhizal networks. New Phytol 151:717–724. https://doi.org/10.1046/j.0028-646x.2001.00216.x
Hallama M, Pekrun C, Lambers H, Kandeler E (2019) Hidden miners – the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 434:7–45. https://doi.org/10.1007/s11104-018-3810-7
Hallama M, Pekrum C, Pilz S, Jarosch KA, Frąc M, Uksa M, Marhan S, Kandeler E (2021) Interactions between cover crops and soil microorganisms increase phosphorus availability in conservation agriculture. Plant Soil 463:307–328. https://doi.org/10.1007/s11104-021-04897-x
Havlin JL, Tisdale SL, Nelson WL, Beaton JD (2017) Soil fertility and fertilizers – an introduction to nutrient management. Perason, Chennai
Hayes JE, Simpson RJ, Richardson AE (2000) The growth and phosphorus utilisation of plants in sterile media when supplied with inositol hexaphosphate, glucose 1-phosphate or inorganic phosphate. Plant Soil 220(1/2):165–174. https://doi.org/10.1023/A:1004782324030
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Hinsinger P (1998) How do plant roots acquire Mineral nutrients? Chemical processes involved in the Rhizosphere. Adv Agron 64:225–265. https://doi.org/10.1016/S0065-2113(08)60506-4
Horváth B, Opara-Nadi O, Beese F (2005) A simple method for measuring the Carbonate Content of soils. Soil Sci Soc Am Jo 69:1066–1068. https://doi.org/10.2136/sssaj2004.0010
International Organization for Standardization (ISO) (2019) Soil Quality - Determination of the Water-Retention Characteristic - Laboratory Methods (ISO 11274:2019) Available via https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjq5J2D-o3_AhUGm_0HHSt0AxYQFnoECAgQAQ&url=https%3A%2F%2Finfostore.saiglobal.com%2Fpreview%2F557774344415.pdf%3Fsku%3D878288_SAIG_NSAI_NSAI_2782562&usg=AOvVaw0-0EpPgV8g9PKCGe3n7FYc. Accessed 24 May 2023
IUSS Working Group WRB (2014) International soil classification system for naming soils and creating legends for soil maps. World reference base for Soil resources 2014. World Soil Resources Reports No. 106. FAO, Rome
Johri AK, Oelmuller R, Dua M, Yadav V, Kumar M, Tuteja N, Varma A, Bonfante P, Persson BL, Stroud RM (2015) Fungal association and utilization of phosphate by plants: success, limitations, and future prospects. Front Microbiol 6:984. https://doi.org/10.3389/fmicb.2015.00984
Kalinova J, Vrchotova N, Triska J (2007) Exudation of Allelopathic substances in Buckwheat (Fagopyrum esculentum Moench). J Agric Food Chem 55(16):6453–6459. https://doi.org/10.1021/jf070795u
Kilian R (2016) Lacy Phacelia (Phacelia tanacetifolia Benth.) A native annual forb for conservation use in Montana and Wyoming. United States Department of Agriculture. Available via https://www.nrcs.usda.gov/plantmaterials/mtpmctn12938.pdf. Accessed 20 Nov 2023
Kim KR, Owens G, Kwon SIK (2010) Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: a rhizobox study. J Environ Sci 22(1):98–105. https://doi.org/10.1016/S1001-0742(09)60080-2
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100(2):263–288. https://doi.org/10.3732/ajb.1200474
Likar M, Bukovnik U, Kreft I, Chrungoo NK, Regvar M (2008) Mycorrhizal status and diversity of fungal endophytes in roots of common buckwheat (Fagopyrum esculentum) and tartary buckwheat (F. Tataricum). Mycorrhiza 18:309–315. https://doi.org/10.1007/s00572-008-0181-6
Maseko ST, Dakora FD (2019) Relationship between acid phosphatase activity and P concentration in organs of Cyclopia and Aspalathus species, and a non-legume of the Cape Floristic Region. J Plant Ecol 12(2):387–392. https://doi.org/10.1093/jpe/rty032
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Myers RL, Meinke LJ (1994) Buckwheat: A multi-purpose, short-season alternative. University of Missouri Extension Bulletin G4306. Available via: http://extension.missouri.edu/publications/DisplayPub.aspx?P=G4306. Accessed 18 Nov 2023
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann EK, Oberson A, Frossard E (eds) Soil Biology. Springer, Berlin Heidelberg, pp 215–243
Niemi RM, Vepsäläinen M (2005) Stability of the fluorogenic enzyme substrates and pH optima of enzyme activities in different Finnish soils. J Microbiol Methods 60:195. https://doi.org/10.1016/j.mimet.2004.09.010
Oburger E, Jones DL, Wenzel W (2011) Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 341(1):363–382. https://doi.org/10.1007/s11104-010-0650-5
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of Soil Analysis Part 2 Chemical and Microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, pp 403–430
Peng Q, Wu M, Zhang Z, Su R, He H, Zhang X (2020) The interaction of arbuscular mycorrhizal fungi and phosphorus inputs on selenium uptake by alfalfa (Medicago sativa L.) and selenium fraction transformation in soil. Front Plant Sci 26(11):966. https://doi.org/10.3389/fpls.2020.00966
Plenchette C, Clermont-Dauphin C, Meynard JM, Fortin JA (2011) Managing arbuscular mycorrhizal fungi in cropping systems. Can J Plant Sci 85(1):31–40. https://doi.org/10.4141/P03-159
Ranganatha ARG, Jyotishi A, Deshmukh MR, Bisen R, Panday AK, Gupta KN, Jain S, Paroha S (2013) Improved technology for maximizing production of Niger. Indian Council of Agricultural Research, Jabalpur. Available via https://icar-iior.org.in/sites/default/files/iiorcontent/pops/niger.pdf. Accessed 18 Nov 2023
Regional economic accounts for agriculture - REAA (2021) Output value, intermediate consumption and gross value added of the agricultural industry by federal provinces in 2021 (at current prices) – in million Euro. Available via https://www.statistik.at/en/statistics/agriculture-and-forestry/agricultural-and-forestry-economy-and-prices/economic-accounts-for-agriculture/regional-economic-accounts-for-agriculture. Accessed 03 May 2023
Rheinheimer DS (2000) Dinâmica do fósforo em sistemas de manejo de solo. Thesis (Doctorate in Agronomy) - Federal University of Rio Grande do Sul, Porto Alegre, 2000
Schilling G, Merbach W, Deubel A, Ležovič G, Ruppel S (1998) Phosphorus availability, root exudates, and microbial activity in the rhizosphere. J Soil Sci Plant Nutr 161(4):465–478. https://doi.org/10.1002/jpln.1998.3581610413
Schwerdtner U, Spohn M (2022) Plant species interactions in the rhizosphere increase maize N and P acquisition and maize yields in intercropping. J Soil Sci Plant Nutr 22:3868–3884. https://doi.org/10.1007/s42729-022-00936-3
Soltangheisi A, Teles APB, Sartor LR, Pavinato PS (2020) Cover cropping may alter legacy Phosphorus Dynamics under Long-Term Fertilizer Addition. Front Environ Sci 8:13. https://doi.org/10.3389/fenvs.2020.00013
Spohn A, Kuzyakov Y (2013) Distribution of microbial- and root-derived phosphatase activities in the rhizosphere depending on P availability and C allocation – coupling soil zymography with 14C imaging. Soil Biol and Biochem 67:106–113. https://doi.org/10.1016/j.soilbio.2013.08.015
Tabatabai MA, Bremmer JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Tang C, Robson AD, Longnecker NE, Greenway H (1993) Physiological responses of lupin roots to high pH. Plant Soil 155:509–512. https://doi.org/10.1007/BF00025095
Ugrenović V, Popović V, Ugrinović M, Filipović V, Mačkić K, Ljubičić N, Popović S, Lakić Ž (2021) Black oat (Avena strigosa Schreb.) Ontogenesis and agronomic performance in organic xropping system and pannonian environments. Agriculture 11(1):55. https://doi.org/10.3390/agriculture11010055
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64(12):5004–5007. https://doi.org/10.1128/AEM.64.12.5004-5007.1998
Vierheiligh H, Lerat S, Piché Y (2003) Systemic inhibition of arbuscular mycorrhiza development by root exudates of cucumber plants colonized by Glomus mosseae. Mycorrhiza 13:167–170. https://doi.org/10.1007/s00572-002-0219-0
Wang L, Nancollas GH (2008) Calcium orthophosphates: crystallization and dissolution. Chem Rev 108(11):4628–4669. https://doi.org/10.1021/cr0782574
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92(4):487–511. https://doi.org/10.1093/aob/mcg164
Wuddivira M, Camps-Roach G (2006) Effects of organic matter and calcium on soil structural stability. Eur J Soil Sci 58(3):722–727. https://doi.org/10.1111/j.1365-2389.2006.00861.x
Zoboli O, Zessner M, Rechberger H (2016) Supporting phosphorus management in Austria: potential, priorities and limitations. Sci Total Environ 565(15):313–323. https://doi.org/10.1016/j.scitotenv.2016.04.171
Acknowledgements
We thank Dr. Karin Hage-Ahmed for providing directions for the mycorrhizal infection rate analysis and Dr. Christiana Staudinger and Harrie Mort for critical proof reading of the final manuscript.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The RecaP project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 956454.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the conceptualization and design of this study. Material preparation, data collection and analysis were performed by Henrique Rasera Raniro, who also wrote the first draft of the manuscript. Jakob Santner provided guidance through the development of the study and feedback on all draft versions. Both authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: N. Jim Barrow.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer . The results expressed in this article are those of the authors only and do not necessarily reflect those of the European Union. The European Union cannot be held responsible for them.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 123 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raniro, H.R., Santner, J. Differential phosphorus acquisition strategies of nine cover crop species grown in a calcareous and a decalcified chernozem. Plant Soil 498, 671–684 (2024). https://doi.org/10.1007/s11104-023-06466-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06466-w