Abstract
Aims
Root system architecture (RSA) plays a crucial role in determining the efficiency of absorbing water in the different soil layers. Studies on the RSA, however, are limited partly because plant roots are found underground and difficult to observe them during plant development. This study aimed to assess the variation in the RSA traits of sorghum landraces at the seedling stage.
Methods
A set of one hundred sixty diverse sorghum genotypes were grown in soil-based rhizotrons and data on nodal root angles (NRA), days to nodal root emergence (DNRE), number of nodal roots (NNR), nodal root length (NRL), fresh root weight (RFW), dry root weight (DRW), root-to-shoot ratio (RSR), fresh shoot weight (FSW), dry shoot weight (DSW), leaf area (LA) were collected.
Results
The analysis of variance revealed the presence of high variation among genotypes for all the studied traits. Repeatability of the RSA traits ranged from 44.8% for RSR to 85.2% for NNR. The wide variation ranging from 16.3° to 53.0° and heritability (63.1%) of the nodal root angles allow the selection of desirable genotypes adapted to drought environments. Several diverse sorghum genotypes with narrow and wide nodal root angles were identified. Genotypes with narrow nodal root angles such as G141, G100, and G63 could be prioritized for use in developing cultivars suitable for dry areas.
Conclusions
This study illustrates the presence of promising sorghum genotypes in terms of RSA traits, which should be utilized for the development of novel cultivars that match cultivation environments differing in water availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorghum (Sorghum bicolor (L.) Moench) is a cereal crop widely grown in drought-stressed areas around the world. It is mainly used as food, feed, and for ethanol production (Qi et al. 2019). It is the fifth most significant cereal crop globally after wheat, rice, maize, and barley and provides food for half a billion people in the semi-arid and arid regions of Asia and Africa, including Ethiopia (Ejeta 2005). Sorghum grows in a very wide range of environmental conditions, such as temperate to tropical climates, low to high altitudes, and waterlogged to drought-stressed environments (Borrell et al. 2021). Even though sorghum is a drought-tolerant crop, which is predominantly grown in arid and semi-arid regions, its production is still affected by drought stress at several developmental stages of the crop as reviewed by Abreha et al. (2022). It has been estimated that yield losses exceed 20 million tons or 20% of rain-fed sorghum production every year in arid and semi-arid regions (Kukal and Irmak 2018). Therefore, screening sorghum genetic resources for drought tolerance is a crucial step to deal with drought stress in arid and semi-arid regions through the development of drought-tolerant cultivars (Rono et al. 2016; Farhadi et al. 2022).
The shoot and root characteristics of crops have been used to enhance the adaptability of crops to drought stressed-environments (Borrell et al. 2020, 2021). Several morphological (high grain yield; root system architecture, above and below ground biomass production; high harvest index and small leaf area), physiological (high stomatal conductance; photosynthetic efficiency, water-use efficiency, stay green, reduced evapotranspiration; leaf cuticular wax and leaf water potential), biochemical (high accumulation of abscisic acid and auxin) and phenological (early vigor, early flowering and maturity) traits are used to increase the ability of plant tolerance to arid places (Sonawane and Cousins 2020; Mace et al. 2012; Blum 2005; Takele 2000; Nxele et al. 2017; Yahaya and Shimelis 2022). Root traits such as fine roots, root length and area, root angle, root length density and root weight are important components in response to drought tolerance which regulate the amount of soil area that a plant can explore for water and nutrient extraction from the soil as well as anchor the plant system (Bucksch et al. 2014). The root system architecture (RSA) refers to the entire or part of the root systems that comprise the root distribution (Bucksch et al. 2014). It is one of the major contributing traits to their ability to extract water and nutrients from the deep horizon of the soil, thereby enabling them to adapt to drought-prone environments. The significance of RSA in terms of its effect on plants’ ability to extract water that contributes to increased grain yield under drought-stress conditions has been reported in sorghum (Mace et al. 2012; Singh et al. 2012). Sorghum root system is characterized by a single seminal root and post embryonic nodal roots. The sorghum seminal root which is emerging directly from the embryo and play an important role only in early growth stage for initial water and nutrient extraction and hence is of little essential in late growth stage or mature sorghum (Singh et al. 2011, 2010). While, numerous nodal roots are develop from the below-ground nodes (above the mesocotyl) of the stem and the first flush of the angle of nodal roots are visible when around five leaves have fully expanded (Singh et al. 2010). The nodal root angle is associated with the spatial distribution of roots of mature sorghum plants and hence with their ability to absorb water in the soil (Singh et al. 2012).
The root angle at the seedling stage of the plant is an RSA trait that has received particular attention in sorghum (Singh et al. 2012, 2011; Demelash et al. 2021) and other crops (Manschadi et al. 2008; Alemu et al. 2021). The nodal root angle of sorghum influences horizontal and vertical root structure and distribution in the soil, thereby contributes to stay-green and grain yield (Mace et al. 2012). Singh et al. (2012) reported that root angles and the level of fine roots have significant influence on yield under drought-stress conditions in sorghum. Narrow and wide root angles have been studied for their effects on water and nutrient extraction ability as well as drought tolerance of crop plants (Manschadi et al. 2008). Sorghum genotypes with narrow root angles were reported to have higher drought tolerance and stay-green characteristics (Mace et al. 2012). This is because plants with longer roots and narrower root angles exploit water and nitrogen more effectively that are often available in deeper soil layers (Manschadi et al. 2008; Kitomi et al. 2015; Truco et al. 2000; Mace et al. 2012). On the other hand, shorter roots with wide root angles allow plants to more effectively extract water and nutrients such as, phosphorous that are available in large amount at shallower depths in the soil (Miguel et al. 2015; Parra-Londono et al. 2018). The nodal root angle is therefore a significant factor in selecting sorghum genotypes for breeding programs designed to improve drought tolerance.
Evaluating phenotypic variation is vital for the identification of genotypes with desirable characteristics for crop improvement through breeding. Several studies have focused on phenotypic variation among different sets of sorghum genotypes in the nodal root angles, number of nodal roots, nodal root length and diameter, as well as root and shoot fresh and dry weights were reported (Singh et al. 2011; Demelash et al. 2021; Bantte et al. 2022). Studies have shown that there is a high genetic diversity in Ethiopian sorghum, which makes it more likely to identify genotypes that display desirable RSA characteristics. In Ethiopia, sorghum is predominantly grown in hot and arid lowlands where insufficient and unpredictable rainfall are among the major abiotic factors that limit its production (Hulluka and Esele 1992). However, research on RSA traits of Ethiopian sorghum is almost non-existent, and no cultivars have been developed with improved RSA traits capable of providing better drought tolerance in harsh environments where Ethiopian sorghum is typically grown. The Ethiopian Biodiversity Institute (EBI) has conserved 9,432 sorghum accessions collected across the country (https://www.ebi.gov.et/biodiversity/conservation/genetic-material-holdings/). The RSA and shoot traits of most of these accessions remain uncharacterized. Therefore, the objectives of this study were to characterize the RSA and shoot traits of Ethiopian sorghum genetic resources, determine the extent and pattern of phenotypic variation, and identify promising genotypes for key RSA and shoot traits for use in sorghum breeding programs.
Materials and methods
Plant materials
A total of 160 accessions were used in this study (Supplementary file 1), which are a subset of the germplasm used in our research published in (Enyew et al. 2021). The accessions were selected to represent different sorghum growing regions and different agro climatic zones. Although Ethiopian Biodiversity Institute (EBI) originally collected the accessions from major sorghum-growing regions in Ethiopia, Melkassa Agricultural Research Center (MARK) has used them for research and breeding purposes, and we acquired these accessions from MARK. They will be referred to as “genotypes” from here on for the sake of simplicity.
Design and assembly of root growth chambers (rhizotrons)
For the root system architecture study of the sorghum genotypes, root growth chambers (rhizotrons) were assembled at the Department of Plant Breeding, SLU, Alnarp. To assemble each rhizotron, two 60 cm × 80 cm transparent 4 mm-thick acrylic plastic sheets, five 60 cm × 1 cm self-adhesive rubber strips with a thickness of 4 mm, and 10 bolts and nuts were used. First, the rubber strips were fixed to one of the plastic sheets at five positions along its width including the two margins so that four equal-sized compartments (each with 60 cm (h) × 18.7 cm (l) × 4 mm (w)) could be formed so as to grow four plants at the same time. Then, the second plastic sheet was placed on top, and the two sheets were screwed together at ten points using bolts and nuts corresponding to the two tips of each of the five rubber strips (see Supplementary file 2 for details). The four compartments of each root rhizotron were then filled with a mixture of dried fine soil and sand in a 2:1 ratio. The average weights of each chamber when it was empty, filled with a mixture of dry fine soil and sand, and after saturating to soil to full capacity were 2.1 kg, 3.0 kg, and 3.4 kg, respectively.
Growth conditions
Before planting the seeds, the rhizotrons were placed in a transparent plastic box that served as a support structure and watered from the top, and then left to drain until the soil reached field capacity. The day after the soil reached field capacity, two seeds were planted per compartment at a depth of three cm. The seeds were planted in a way that their embryos face the wall of the rhizotron so that the roots can be clearly visible through the transparent plastic wall as they grow. Three days after germination, one of the two seedlings in each compartment was removed. The plants were grown in a greenhouse where day/night temperatures and average relative humidity were set to 28/22°C and 65%, respectively. The layout of the rhizotrons containing the growing plants representing the 160 genotypes was a completely randomized design with three replications. The three replications were achieved by growing the plants at three different times (planting dates) in the same greenhouse.
Data collection
All the data were collected after three weeks at five to six leaf stages of the plants except days to nodal root emergence (DNRE), which was recorded as the date of the first flash of nodal root emergence. For nodal root angle (NRA) measurement, the rhizotrons were removed from the box and held vertically adjacent to a ruler, and images of the root system that was visible through the transparent wall of the rhizotrons were taken using a Canon EOS 1300D digital camera with 18-megapixel resolution. The images were used to measure the angle of each of the first pair of nodal roots with a reference to a perpendicular line to the main stem at a 3 cm distance from the base of the stem using RootNav software version 1.8.1 (Pound et al. 2013). The nodal root angle of each plant was then recorded as the average value of the angles measured from the images taken on the left and right sides of each plant on both sides of the rhizotron.
After images were taken, the rhizotrons were disassembled and the roots were carefully washed and separated from the soil. The nodal roots were then placed horizontally and pictures were taken with a digital camera fixed on a tripod. The number of nodal roots (NNR) of each plant was counted and the average nodal root length (NRL) of each plant was determined using ImageJ software (Abràmoff et al. 2004). Immediately after taking images, the fresh root weight (FRW) of each plant was measured using a high precision scale. This was followed by drying the roots at 60°C for 3 days in an oven and determining the dry root weight (DRW). The leaf area (LA) of each plant was calculated by measuring the largest leaf length and width and then multiplying the obtained value by 0.69, as described by (Lafarge et al. 2002). The fresh shoot weight (FSW) of each plant was measured by cutting the shoots of each plant at the base of the stem while the dry shoot weight was determined after drying the shoots at 60°C for 3 days in an oven. The dry root-to-shoot ratio (RSR) was then calculated by dividing DRW by DSW.
Data analysis
All recorded data were subjected to analysis of variance (ANOVA). Analyses of descriptive statistics and repeatability (H2) of each trait were conducted with R software (R 2022). Phenotypic means of the genotypes were determined for each trait and their significant differences among genotype means were evaluated using the least significant difference (LSD) test (p < 0.05). These means were also used to perform correlation, principal component, and cluster analyses. The correlation analysis between variables were performed by psych package (Revelle 2017) of R software. The prcomp function of the factoextra package (Kassambara and Mundt 2017) of R software was used to perform principal component analysis. For cluster analysis, the Euclidean distance was calculated with the dist function of the cluster package (Kassambara and Mundt 2017) of R software while the neighbor-joining tree was generated with MEGA software (Kumar et al. 2018).
Results
Phenotypic variation and heritability
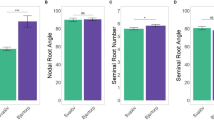
The analysis of variance (ANOVA) revealed highly significant (p < 0.001) variation among genotypes for all studied RSA and shoot traits (Table 1). Wide variation was observed in NRA ranging from 16.3° to 53.0° with a mean of 34.9° (Table 1; Fig. 1). DNRE ranged from seven to 15 days with a mean of 11 days. The average NNR was 6.0 with individual values ranging from two to 11. The genotypes showed wide variation in NRL, ranging from 33.7 cm to 269.1 cm with a mean of 119.1 cm. The FRW and FSW ranged from 0.6 and 0.5 g to 5.3 and 2.5 g, with mean values of 2.3 and 2.5 g, respectively. The RSR ranged from 0.18 to 3.9 with a mean value of 1.0. The DRW varied from 0.05 to 0.75 g with a mean of 0.28 g while the average DSW was 0.35 g with individual values ranging from 0.12 to 0.83 g. The average leaf area was 10.5 cm2 with individual values ranging from 3.1 to 21.5 cm2 (Table 1). The repeatability (H2) of the RSA traits was moderate for RSR (44.8%), NRL (48.4), DNRE (50.9%), and DRW (55.0%) while it was high for LA (61.3%), NRA (63.1), FRW (64.5), DSW (70.2), FSW (74.7%), and NNR (85.2) (Table 1).
A photograph of rhizotrons in rectangular plastic boxes containing sorghum seedlings at 21 days after planting (A); photographs of nodal roots of a genotype with a narrow nodal root angle (B), and a genotype with a wide root angle (C) taken through the transparent walls of the rhizotrons, the yellow lines demonstrate the angles; a photograph of nodal roots taken through the wall of a rhizotron revealing variation in their angles of growth; D a photograph of detached nodal roots of two genotypes showing variation in the number of nodal roots and nodal root length (E)
Mean performance of genotypes for RSA traits
The nodal root angles of 84 genotypes were above the mean whereas those of 76 genotypes were below the mean. The top three genotypes with narrow root angles were G141 (16.3°), G100 (18.9°), and G63 (19.3°) and those with wider root angles were G1 (53.0°), G16 (52.4°), and G51 (51.1°) (Table 2). Similarly, days to nodal root emergence of 84 genotypes was above the mean while that of 76 genotypes was below the mean. Genotypes G82, G49, and G143 showed the earliest nodal root emergence seven to eight days after planting. In contrast, G40, G144, and G2 were the latest requiring 14 to 15 days for the emergence of nodal roots. The number of nodal roots (NNR) of 92 and 69 genotypes was above and below the mean values, respectively. G104, G141, and G160 came on top for this trait with 10 to 11 nodal roots, while G79, G124, and 92 had the lowest number of nodal roots (2 to 3). With regard to nodal root length (NRL), 68 and 92 genotypes scored above and below the mean values, respectively. G141, G151, and G160 were top ranking for this trait (244–296 cm) while G79, G157, and G4 were bottom ranking (34–38 cm) (Table 2).
With regard to FRW, the top three genotypes were G86, G150, and G12 while the bottom three were G83, G79, and G92. The dry root weights (DRW) of 70 genotypes were above the mean while 90 genotypes had DRW below the mean. Genotypes G158, G39, and G54 had the highest RDW (0.7–0.8 g). The RSR of the genotypes ranged from 0.18 (for genotype G43) to 3.9 (for genotype G60). The top three accessions were G77, G155, and G129 for FSW while G2, G87, and G86 were the top three accessions for DSW. With regard to DSW, 60 genotypes scored values above the mean while 90 genotypes scored below the mean. In terms of leaf area (LA), G129, G59, and G151 ranked at the top, whereas G15, G6, and G79 ranked at the bottom. In all traits, the top three genotypes differ significantly from the bottom three genotypes (Table 2).
Correlation among the RSA traits
The correlation analysis showed that there were significant positive and negative associations among RSA traits (Fig. 2). Nodal root angle showed significant negative correlations with all RSA traits except with DNRE and RSR. Similarly, the DNRE showed significant negative correlations with all the studied traits except the RSR and NRA. The NRL exhibited significant positive correlations with the NNR (r = 0.75), FRW (r = 0.61), DRW (r = 0.29), FSW (r = 0.74), DSW (r = 0.64) and LA (r = 40). Similarly, the NNR showed significant positive correlations with FRW, DRW, FSW, DSW, and LA. On the other hand, RSR showed significant negative correlations with NRL, NNR, FSW, DSW, and LA. Highly significant positive correlations were observed between FRW, DRW, FSW, and DSW. LA also showed significant positive corrections with FRW, DRW, FSW, and FSW (Fig. 2).
Variation and correlation between the nine root system architecture and shoot traits. Histograms for all traits are displayed along the diagonal. To the left and below the diagonal are scatter plots comprising measured individuals from 160 genotypes. The red line through the scatter plot represents the line of best fit. NRA = nodal root angle, DNRE = days to nodal root emergence, NNR = number of nodal roots, NRL = nodal root length, RFW = fresh root weight, DRW = dry root weight, RSR = root to shoot ratio, FSW = fresh shoot weight, DSW = dry shoot weight, LA = leaf area, *** = Significant at 0.001 significance level, ** = Significant at 0.01 significance level, * = Significant at 0.05 significance level
Principal component and cluster analysis
The principal component analysis of the 160 sorghum genotypes based on the correlation matrix of RSA traits yielded the eigenvalues and eigenvectors (Fig. 3 and Supplementary file 3). Ten principal components explained the total variation. The first two principal components (PC1 and PC2), with eigenvalues greater than one cumulatively explained 62.9% of the total variation among the genotypes with regard to the studied traits. The first principal component accounted for 46.64% of the total variation while the second component accounted for 16.5% of the total variation (Figs. 3A and 4). The third and fourth principal components explained 9.5% and 8.5% of the total variation, respectively. Five traits, namely fresh shoot weight, dry shoot weight, nodal root length, number of nodal roots, and fresh root weight were major contributors to the variation in PC1 (Figs. 3B and 4). While root-shoot-ratio and dry root weight were major contributors to the variation in PC2. The days to nodal root emergence and nodal root angle had the highest contribution to the variation in PC3 and PC4, respectively.
A Scree plot showing the variation each principal component captures from the RSAdata and (B) contributions of variables to principal components. The size and color of the dots are proportional to the contribution of the traits for each principal components. NRA = nodal root angle, DNRE = days to nodal root emergence, NNR = number of nodal roots, NRL = nodal root length, RFW = fresh root weight in gram, DRW = dry root weight, RSR = root to shoot ratio, FSW = fresh shoot weight, DSW = dry shoot weight, LA = leaf area
Principal component analysis (PCA) showing the clustering pattern of 160 Ethiopian sorghum genotypes regarding variation in root system architecture traits. An individual that is on the same side of a given variable has a high value for this variable; an individual that is on the opposite side of a given variable has a low value for this variable. Supplementary file 1 can be used to access the genotype code and passport data of sorghum genotypes
The PCA biplot shows the genotypes with RSA traits explained by the first two dimensions (Fig. 4). Among the traits, DRW, RSR, FSW, DSW, FRW, NRL and NNR showed the highest contribution to the variation that responsible for discriminating genotypes under the biplot of PC1 and PC2 (Fig. 4). The PCA biplot indicated that the distant/diverging genotypes from the major genotypes such as G141 had a narrow root angle, the highest number of nodal roots and length; G158 had high dry root weight (0.98 g); G39 had the highest dry root weight; G60 had the highest root to shoot ratio (0.29 g); G157 had the lowest nodal root length and G79 had the lowest shoot fresh weight, G83 had the lowest fresh and dry root weight, G31had the lowest root to shoot ratio and G39 had the highest root dry weight and root to shoot ratio (Fig. 4). The principal component analysis did not clearly cluster genotypes based on the proximity of their original collected geographical locations (Supplementary file 4).
A neighbor-joining tree was generated through cluster analysis of the 160 sorghum genotypes based on the phenotypic data collected on the RSA and shoot traits. The cluster analysis grouped the genotypes into four clusters. Cluster I, II, II, and IV comprised 13, 11, 36, and 100 genotypes respectively, accounting for 8.1%, 6.9%, 22.9%, and 62.5% of the 160 genotypes (Fig. 5). Cluster I represents genotypes with the highest values for NRL, FRW, DSW, and LA, and the lowest value for NRA on average (Supplementary file 5). Cluster II was characterized by genotypes with the highest values for the NNR, FSW, and DSW on average. Cluster III was characterized by genotypes with the highest values for NRA, DNRE, and RSR, and the lowest values for all other target traits on average. Representing the majority of the genotypes, Cluster IV comprised genotypes that, on average, did not display the highest or lowest values for the target traits (Supplementary file 5). The top genotypes with narrow root angles, G63, G141, and G152, which were originally collected from the Tigray and Amhara regions, were grouped under Cluster I. Among genotypes that came on top in terms of wide root angles, G1 was originally collected from the Amhara region, whereas G16 and G51 were originally collected from the Oromia region. Genotypes representing improved varieties were grouped under Clusters II and III. Cluster analysis revealed poor clustering patterns of genotypes according to their geographical region of origin (Fig. 5).
A neighbor-joining tree of the 160 sorghum genotypes generated based on phenotypic data of nine root architecture and shoot traits revealing four clusters. Genotypes denoted by the same color labels belong to the same geographic region, Supplementary file 1 can be used to access the genotype code and passport data of sorghum genotypes
Discussion
Ethiopian sorghum genotypes showed wide variations in root system architecture traits
The presence of high genetic variation among sorghum landraces has been reported in various previous studies (Demelash et al. 2021; Bantte et al. 2022; Enyew et al. 2022a, b, 2021). Sorghum breeding programs could benefit from its extensive genetic diversity, as it facilitates cultivar development for cultivation under both diverse and specific agro-climatic conditions (Shelbourne 1992). It is crucial to study the genetics of root system architecture to better understand crops' adaptation mechanisms to abiotic stresses, such as drought. In this regard, the genetic component of variation in root traits is vital to enhance crop performance and adaptation to different environmental conditions, through improving plant root system architecture (Lynch 2007). The present study, based on 160 genotypes from diverse regions in Ethiopia, revealed large genetic variations in root system architecture and shoot traits that may be of substantial value for improving sorghum adaptation to various environmental conditions. This study revealed highly significant differences in root system architecture and shoot traits between sorghum genotypes that showed moderate to high repeatability. Nodal root angle is among the targeted traits of the present study that showed large variation and high repeatability (H2 = 63.2%) which is in line with the results obtained in previous studies on sorghum reporting repeatability ranging from 66.2% to 96% (Mace et al. 2012; Joshi et al. 2017; Tebeje et al. 2020; Bantte et al. 2022). However, moderate repeatability was reported for this trait in some other studies, including in the work of Singh et al. (2011) where 46.6% repeatability was reported.
The root system architecture can play a crucial role in the ability of a crop to cope with moisture stress conditions (Manschadi et al. 2006). In this study, the nodal root angle showed wide variation, ranging from 16.3° to 53.0° with a mean value of 34.9°. This is in agreement with previous studies in sorghum, which ranged from about 15º to 50º for 44 sorghum inbred lines (Singh et al. 2011), and 17.6° to 41.3° for a backcross nested association mapping population, 16.0° to 42.0° for the advanced hybrids (Joshi et al. 2017). Genotypes with narrow root angles were reported to have a higher ability to access water stored deep in the soil in sorghum and wheat (Manschadi et al. 2008; Singh et al. 2012). Studies have shown that sorghum genotypes with narrow nodal root angles and wheat genotypes with seminal root angles at a seedling stage developed a narrow, compact, and deeper root system at later developmental stages (Manschadi et al. 2008; Singh et al. 2012; Rich et al. 2020). This indicates that screening sorghum genotypes for root angles at the seedling stage is an efficient and economical approach to identify genotypes with narrow nodal root angles for use in sorghum breeding programs. Sorghum genotypes with a narrow root angles were reported to have higher drought tolerance and stay-green traits than genotypes with wide root angles (Mace et al. 2012) and could positively affect grain yield in drought-stressed environments (Singh et al. 2012). In the present study, the top three genotypes with narrow root angles were G141 (16.3°), G100 (18.9°), and G63 (19.3°). More interestingly, G141 and G100 showed high chlorophyll content and grain yield, respectively in our previous field experiment (Enyew et al. 2022a, 2021). Therefore, they could be prioritized for use in the development of cultivars for hot and dry lowland areas where erratic rainfall and prolonged moisture stress are common occurrences.
Among the genotypes with wide nodal root angles, G1 (53.0°), G16 (52.4°), and G51 (51.1°) were the top three. Plants with wide root angles have increased root absorption areas horizontally in the topsoil, resulting in improved water extraction capabilities in wide or skip-row agriculture (Singh et al. 2011; Ali et al. 2015). In addition, genotypes with wider root angles and shallower root systems may be able to exploit water obtained through light rainfall more effectively (Liao et al. 2006). Therefore, in environments where sorghum largely depends on stored water in deeper soil, genotypes with wide nodal roots may have limited access to water and nutrients such as nitrogen in deeper soil layers. On the other hand, wide nodal roots may have an increased contribution to water extraction from the shallow soil layers that get wet through frequent and substantial in-season rainfall. The density of roots in the upper soil layers is an essential trait for the absorption of nutrients, such as phosphorus in the top soil layer (Manske et al. 2000). (Parra-Londono et al. 2018) also reported that sorghum genotypes with a compact and bushy but shallow root system provide better potential adaptation to agricultural fields with low phosphorus. Therefore, the identified sorghum genotypes with wide root angles could match the descriptions of an ideotype for the exploitation of water and nutrients, such as phosphorus available in the topsoil layer. Cultivars that will be developed on such sorghum genotypes are suitable in environments where water is sufficiently available through rainfall or irrigation during the crop-growing seasons.
In addition to nodal root angle, the number of nodal roots and length are also factors contributing to the adaptation of plants to drought stress. In this study, a large variation was observed in root number and length. Variation in these traits at seedling stage has been reported in sorghum (Demelash et al. 2021) and other crops, such as wheat (Manschadi et al. 2008; Alemu et al. 2021) and barley (de Dorlodot et al. 2007). Root length is one of the most important traits for screening crop genotypes for drought-stress tolerance due to the fact that plants with deep roots provide higher yields under drought-stress conditions by developing roots over long distances and accessing water from the deep soil layers (Li et al. 2022). Therefore, sorghum genotypes identified in this study combining characteristics of a higher number of long nodal roots and a narrower root angle, such as genotypes G141, G160, G150, and G153 can be considered ideal for developing cultivars suitable for dry lowland areas where plants rely heavily on subsoil water.
Nodal root angle showed moderate degree of association with root and shoot traits
In the present study, correlation analysis revealed significant positive and negative correlations between RSA and shoot traits. A significant negative correlation was found between nodal root angle and RSA and shoot traits, such as the number of nodal roots, the length of nodal roots, and the biomass of shoots and roots. This indicates that genotypes with narrower nodal root angles are likely to develop a higher number of nodal roots that are longer than average having a higher shoot and root biomass. This is probably because these traits are controlled by the same set of genes. The correlation of narrow root angles with a longer and higher number of roots in this and previous studies in sorghum as well as in wheat (El Hassouni et al. 2018) signifies that genotypes with narrow root angles are more effective for utilization of stored soil moisture by developing deep penetrating roots, thereby improving grain yields under drought stress (Yu et al. 2015; El Hassouni et al. 2018; Demelash et al. 2021; Shinde et al. 2017). However, the lack of correlation or weak correlation between nodal root angles and shoot traits was also reported (Mace et al. 2012; Bantte et al. 2022). The difference may be due to differences in the genetic architecture of the genotypes used, which needs to be further investigated.
In this study, highly significant positive correlations were observed for fresh and dry root weights versus fresh and dry shoot weights. Similarly, leaf area showed significant positive correlation with fresh and dry root and shoot weights. This is in agreement with previous studies on sorghum and other crops (Kunasekarn et al. 2017; Alemu et al. 2021; Bantte et al. 2022). The strong association between root and shoot biomass traits observed in this and previous studies suggests that they may be controlled by the same set of genes (López-Castaneda and Richards 1994).
Principal component and cluster analyses revealed most diverging genotypes for the RSA and shoot traits
In PCA, the number of principal components retained can be determined based on eigenvalues (Kaiser 1961). PCs with eigenvalues above one represent large variances (Tetteh et al. 2019). In the present study, the first two PCs with eigenvalues greater than 1.0 accounted for 62.9% of the total variance, with PC1 accounting for 46.64% of the total variation and PC2 contributing 16.5% of the total variation. The PCA analysis revealed most diverging genotypes for the RSA and shoot traits, these pattern helps to select specific genotypes with particular traits for sorghum breeding programs.
In the cluster analysis, some genotypes that have similar mean values for one or more traits were placed in the same cluster, which helps to identify promising genotypes based on the mean values of genotypes in the same cluster (Seetharam and Ganesamurthy 2013). Genotypes in the different clusters exhibiting similar desirable characteristics are presumably genetically more distinct from each other, and hence provide opportunities to select distinct genotypes for crossbreeding. Crossbreeding could lead to higher heterotic groups of shoot and root traits for crop improvement, which facilitates the development of cultivars that can overcome different constraints of sorghum production in moisture-stressed areas. Similar to PCA, cluster analysis showed the presence of high variation in several root traits among genotypes. In the present study, genotypes grouped under Cluster I had the highest average values of NRL, DRW, DSW, RSR, and LA. Cluster I included genotypes G63, G141 and G152, which had the longest nodal roots and narrow root angles. These genotypes were originally collected from major sorghum growing areas in the Tigray and Amhara regions of Ethiopia. According to Singh et al. (2012), different sorghum genotypes with long nodal roots and narrow angles at the seedling stage have a high genetic potential for drought tolerance. Genotypes with more roots in deeper soil layers produced 28 to 42% higher yields, on average, which is most likely due to their ability to extract deep soil moisture during grain-filling stage (Lynch and Wojciechowski 2015). Genotypes G1, G16, and G51 with wider root angles from the Oromia and Amhara regions were placed under Cluster II. The clusters revealed through cluster analysis were found to be composed of genotypes collected from different geographical regions. This indicated a wide geographical distribution of sorghum genotypes having diverse root system architecture and shoot characteristics of agronomic significance.
Conclusions
Sorghum genotypes representing the crop's gene pool in Ethiopia exhibited large variations in root system architecture and shoot traits that can be exploited to improve the crop. Root system architecture and shoot traits showed moderate to high repeatability. Among the root system architecture and shoot traits targeted in this study, the nodal root angle is a key trait due to its effect on the distribution of plant roots in the soil, thereby determining the efficiency of the plant’s water and nutrient-extracting ability from the soil at different depths. The large variation and heritability of the nodal root angle facilitate the selection of desirable genotypes adapted to water-stressed environments, which can be used in sorghum breeding programs for the development of cultivars suitable for such environments. Several sorghum genotypes with diverse genetic backgrounds, which had narrow and wide nodal root angles have been identified in the present study. They should be utilized as a source of valuable root system architecture alleles for developing new cultivars for drought-stress tolerance. Moreover, this study demonstrates that simple and affordable rhizotrons are efficient to study plant root system architecture, and could be used for screening a larger number of sorghum genotypes and other crops with further modifications to accommodate more plants.
Data availability
The dataset used in this study can be found in the article/Supplementary Material. Further inquiries about the data can be directed to the corresponding author.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11(7):36–42
Abreha KB, Enyew M, Carlsson AS, Vetukuri RR, Feyissa T, Motlhaodi T, Ng’uni D, Geleta M (2022) Sorghum in dryland: morphological, physiological, and molecular responses of sorghum under drought stress. Planta 255(1):1–23
Alemu A, Feyissa T, Maccaferri M, Sciara G, Tuberosa R, Ammar K, Badebo A, Acevedo M, Letta T, Abeyo B (2021) Genome-wide association analysis unveils novel QTLs for seminal root system architecture traits in Ethiopian durum wheat. BMC Genomics 22(1):1–16
Ali ML, Luetchens J, Nascimento J, Shaver TM, Kruger GR, Lorenz AJ (2015) Genetic variation in seminal and nodal root angle and their association with grain yield of maize under water-stressed field conditions. Plant Soil 397(1):213–225
Bantte K, Menamo TM, Borrell AK, Mace E, Jordan DR, Tao Y, Hunt C (2022) Genetic dissection of root architecture in Ethiopian sorghum landraces. https://www.researchsquare.com/article/rs-2159601/v1
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56(11):1159–1168
Borrell A, Oosterom Ev, George-Jaeggli B, Vadez V, Singh V, Hammer G (2020) Physiology of growth, development and yield. In: Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World. Springer, pp 127–155. https://doi.org/10.1007/978-981-15-8249-3_6
Borrell A, van Oosterom E, George-Jaeggli B, Rodriguez D, Eyre J, Jordan DJ, Mace E, Singh V, Vadez V, Bell M (2021) Sorghum. In: Crop physiology case histories for major crops. Elsevier, pp 196–221. https://doi.org/10.1016/B978-0-12-819194-1.00005-0
Bucksch A, Burridge J, York LM, Das A, Nord E, Weitz JS, Lynch JP (2014) Image-based high-throughput field phenotyping of crop roots. Plant Physiol 166(2):470–486
de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12(10):474–481
Demelash H, Tadesse T, Menamo T, Menzir A (2021) Determination of root system architecture variation of drought adapted sorghum genotypes using high throughput root phenotyping. Rhizosphere 19:100370
Ejeta G (2005) Integrating biotechnology, breeding, and agronomy in the control of the parasitic weed Striga spp in sorghum. In the wake of the double helix: from the green revolution to the gene revolution, Bologna Bologna: 239–251. https://dista.unibo.it/doublehelix/proceedings/SECTION_III/HELIX%20pp%20239-251.pdf
El Hassouni K, Alahmad S, Belkadi B, Filali-Maltouf A, Hickey L, Bassi F (2018) Root system architecture and its association with yield under different water regimes in durum wheat. Crop Sci 58(6):2331–2346
Enyew M, Feyissa T, Geleta M, Tesfaye K, Hammenhag C, Carlsson AS (2021) Genotype by environment interaction, correlation, AMMI, GGE biplot and cluster analysis for grain yield and other agronomic traits in sorghum (Sorghum bicolor L. Moench). Plos one 16(10):e0258211
Enyew M, Carlsson AS, Geleta M, Tesfaye K, Hammenhag C, Seyoum A, Feyissa T (2022a) Novel sources of drought tolerance in sorghum landraces revealed via the analyses of genotype-by-environment interactions. Front Plant Sci: 5022
Enyew M, Feyissa T, Carlsson AS, Tesfaye K, Hammenhag C, Geleta M (2022b) Genetic diversity and population structure of sorghum (Sorghum bicolor (L.) Moench) accessions as revealed by single nucleotide polymorphism markers. Front Plant Sci: 3110. https://doi.org/10.3389/fpls.2021.799482
Farhadi A, Paknejad F, Golzardi F, Ilkaee MN, Aghayari F (2022) Effects of limited irrigation and nitrogen rate on the herbage yield, water productivity, and nutritive value of sorghum silage. Commun Soil Sci Plant Anal 53 (5):576–589. https://doi.org/10.1080/00103624.2021.2017959
Hulluka M, Esele J (1992) Sorghum diseases in eastern Africa. Sorghum and millets diseases: a second world review 502(324):21. https://oar.icrisat.org/994/1/RA_00223.pdf#page=31
Joshi DC, Singh V, Hunt C, Mace E, van Oosterom E, Sulman R, Jordan D, Hammer G (2017) Development of a phenotyping platform for high throughput screening of nodal root angle in sorghum. Plant Methods 13(1):1–12
Kassambara A, Mundt F (2017) Package ‘factoextra’. Extract and visualize the results of multivariate data analyses 76(2)
Kitomi Y, Kanno N, Kawai S, Mizubayashi T, Fukuoka S, Uga Y (2015) QTLs underlying natural variation of root growth angle among rice cultivars with the same functional allele of DEEPER ROOTING 1. Rice 8(1):1–12
Kukal MS, Irmak S (2018) Climate-driven crop yield and yield variability and climate change impacts on the US Great Plains agricultural production. Sci Rep 8(1):1–18
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Kunasekarn R, Kulandaivelu G, Arumugam Y (2017) Correlation analysis for shoot/root parameters under polyethylene glycol (PEG) induced water stress in sorghum (Sorghum bicolor (L.) Moench) genotypes. Int J Chem Stud 5(6):389–399
Lafarge T, Broad I, Hammer G (2002) Tillering in grain sorghum over a wide range of population densities: identification of a common hierarchy for tiller emergence, leaf area development and fertility. Ann Bot 90(1):87–98
Li B, Zhang X, Morita S, Sekiya N, Araki H, Gu H, Han J, Lu Y, Liu X (2022) Are crop deep roots always beneficial for combating drought: A review of root structure and function, regulation and phenotyping. Agric Water Manag 271:107781
Liao M, Palta JA, Fillery IR (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Aust J Agric Res 57(10):1097–1107
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55(5):493–512
Lynch JP, Wojciechowski T (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. J Exp Bot 66(8):2199–2210
Mace E, Singh V, Van Oosterom E, Hammer G, Hunt C, Jordan D (2012) QTL for nodal root angle in sorghum (Sorghum bicolor L. Moench) co-locate with QTL for traits associated with drought adaptation. Theor Appl Genet 124(1):97–109
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33(9):823–837
Manschadi AM, Hammer GL, Christopher JT, Devoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303(1):115–129
Manske G, Ortiz-Monasterio J, Van Ginkel M, Gonzalez R, Rajaram S, Molina E, Vlek P (2000) Traits associated with improved P-uptake efficiency in CIMMYT’s semidwarf spring bread wheat grown on an acid Andisol in Mexico. Plant Soil 221(2):189–204
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167(4):1430–1439
Nxele X, Klein A, Ndimba BK (2017) Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S Afr J Bot 108:261–266
Parra-Londono S, Kavka M, Samans B, Snowdon R, Wieckhorst S, Uptmoor R (2018) Sorghum root-system classification in contrasting P environments reveals three main rooting types and root-architecture-related marker–trait associations. Ann Bot 121(2):267–280
Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T (2013) RootNav: navigating images of complex root architectures. Plant Physiol 162(4):1802–1814
Qi G, Li N, Sun XS, Wang D (2019) Overview of sorghum industrial utilization. Sorghum: State Art Future Perspetives 58:463–476
RCT (2022). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Revelle WR (2017) psych: Procedures for personality and psychological research. https://personality-project.org/r/psych/
Rich SM, Christopher J, Richards R, Watt M (2020) Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J Exp Bot 71(16):4751–4762
Rono JK, Cheruiyot EK, Othira JO, Njuguna VW, Macharia JK, Owuoche J, Oyier M, Kange AM (2016) Adaptability and stability study of selected sweet sorghum genotypes for ethanol production under different environments using AMMI analysis and GGE biplots. Sci World J. https://doi.org/10.1155/2016/4060857
Seetharam K, Ganesamurthy K (2013) Characterization of sorghum genotypes for yield and other agronomic traits through genetic variability and diversity analysis. Electron J Plant Breed 4(1):1073–1079
Shelbourne C (1992) Genetic gains from different kinds of breeding population and seed or plant production population. S Afr for J 160(1):49–65
Shinde M, Awari V, Patil V, Gadakh S, Nirmal S, Dalvi U, Andhale L (2017) Root traits and its correlation with grain yield of rabi sorghum genotypes in phule root box structure under receding soil moisture condition. Int J Curr Microbiol Appl Sci 6(3):977–981
Singh V, van Oosterom EJ, Jordan DR, Messina CD, Cooper M, Hammer GL (2010) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333(1–2):287–299
Singh V, van Oosterom EJ, Jordan DR, Hunt CH, Hammer GL (2011) Genetic variability and control of nodal root angle in sorghum. Crop Sci 51(5):2011–2020
Singh V, van Oosterom EJ, Jordan DR, Hammer GL (2012) Genetic control of nodal root angle in sorghum and its implications on water extraction. Eur J Agron 42:3–10
Sonawane BV, Cousins AB (2020) Mesophyll CO2 conductance and leakiness are not responsive to short-and long-term soil water limitations in the C4 plant Sorghum bicolor. Plant J 103(4):1590–1602
Takele A (2000) Seedling emergence and of growth of sorghum genotypes under variable soil moisture deficit. Acta Agron Hung 48(1):95–102
Tebeje A, Bantte K, Matiwos T, Borrell A (2020) Characterization and association mapping for drought adaptation in Ethiopian sorghum (Sorghum bicolor (L.) Moench) germplasm. Vegetos 33(4):722–743
Truco M, Randall L, Bloom A, St Clair D (2000) Detection of QTLs associated with shoot wilting and root ammonium uptake under chilling temperatures in an interspecific backcross population from Lycopersicon esculentum× L. hirsutum. Theor Appl Genet 101(7):1082–1092
Yahaya MA, Shimelis H (2022) Drought stress in sorghum: Mitigation strategies, breeding methods and technologies—A review. J Agron Crop Sci 208(2):127–142
Yu P, Li X, White PJ, Li C (2015) A large and deep root system underlies high nitrogen-use efficiency in maize production. PLoS ONE 10(5):e0126293
Acknowledgements
The authors acknowledge the Swedish International Development Cooperation Agency (Sida) for financing this research. We are grateful to Melkassa Agricultural Research Center and Ethiopian Biodiversity Institute for providing sorghum germplasm used in this study. We would also like to thank the Institute of Biotechnology, Addis Ababa University and Department of Plant Breeding, Swedish University of Agricultural Sciences, for technical support during the study.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This research work was financially supported by the Swedish International Development Cooperation Agency (Sida) and Research and Training Grant awarded to the Addis Ababa University and the Swedish University of Agricultural Sciences (AAU-SLU Biotech; https://sida.aau.edu.et/index.php/biotechnology-phd-program/; accessed on January 01, 2023) and by the Royal Physiographic Society of Lund (Fysiografen) project #26299000.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the experiment. ME conducted the experiment, analyzed the data and wrote the draft manuscript. MG, ASC, TF, CH, KT and AS reviewed the manuscript. All authors read and approved the submission of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Yinglong Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Enyew, M., Geleta, M., Feyissa, T. et al. Sorghum genotypes grown in simple rhizotrons display wide variation in root system architecture traits. Plant Soil (2023). https://doi.org/10.1007/s11104-023-06373-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-06373-0