Abstract
Background and aims
Selenium (Se)-biofortified foods are able to compensate for Se deficient diets, but the proportion of different Se species varies among plant species, and the bioavailability of Se species also varies. This study aims to examine the Se species composition of four vegetables.
Methods
The effect of Se-enriched irrigation water on the concentrations of Se species in the edible parts of cabbage, carrots, tomatoes and green peas grown on different soil types was investigated and quantified using HPLC-ICP-MS with either standard addition or isotope dilution.
Results
Cabbage leaf and carrot root contained a high proportion (88 and 92%, respectively) of selenate (SeVI), while in tomato fruit and green pea seed selenomethionine (SeMet) was found in the highest proportion (33% and 48%, respectively), but elemental Se was also detected. The elemental Se concentration of peas was significantly higher on sandy soil (2.29 mg kg−1) than on sandy silt (1.38 mg kg−1) or silt soil (1.34 mg kg−1). In 100 g of fresh edible parts, the largest amount of Se species was found in cabbage leaves, but organic Se in the form of SeMet was the most abundant in green peas, being significantly higher than in carrots or tomatoes. Tomatoes contained a nearly three-fold ratio of organic Se to inorganic Se, the latter being mostly comprised of the elemental form, which is unusually high in vegetables. The highest rate of unknown Se species exceeded 66% in the case of peas, while the highest identification rate (> 95%) of Se could be achieved in tomato.
Conclusion
Tomatoes converted the absorbed Se into organic Se in the highest ratio, while green peas had the highest amount of organic Se in fresh biomass, so biotransformation was the most effective for these vegetables without any significant biomass reduction. These vegetables may be adapted to soils with a higher Se content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Greater emphasis should be placed on the appropriate intake of micronutrients, because for farmers nutritional value often takes a backseat compared with crop yield (Lončarić et al. 2021). Selenium (Se) is an essential trace element for both humans and animals. It maintains health via antioxidant effects and cardiovascular benefits, and also supports the immune system and the reproductive organs (Rayman 2012; Koszta et al. 2012; Tóth and Csapó 2018). Inadequate Se intake can lead to serious diseases, which in extreme cases may even be fatal (Hartikainen 2005).
The daily recommended dietary allowance of Se varies considerably across countries and regions. It ranges from 25 to 60 µg day−1 for adult women and from 30 to 75 µg day−1 for adult men, a value of 55 µg day−1 being recommended in the USA and Canada (Fairweather-Tait et al. 2011). In the EU, 70 µg day−1 is recommended for adults and 15 µg day−1 for children aged one to three years, which increases to 70 µg day−1 for adolescents aged 15–17 years. For lactating women an intake of 85 µg day−1 is recommended to cover the amount of Se secreted in breast milk (EFSA NDA Panel, 2014). The tolerable upper intake level is 255 µg Se day−1 for adult men and women (EFSA NDA Panel et al. 2023).
However, the daily intake falls short of these values in many areas of the world. The Se supply of soils may vary from very low to toxic levels even within countries, as reported in the USA, China, Canada, Venezuela, India, Japan and the UK. In North America, for example, the Pacific Northwest and the eastern US are low in Se, but the northern Great Plains and the prairie regions of Canada are rich in Se (McNeal and Balistrieri 1989). Se-deficient nutrition affects a larger area of the world than excessive selenium intake (Pyrzynska 2009). Se deficiency is characteristic of Australia, New Zealand and some areas of Africa, such as the Sub-Saharan region (Gissel-Nielsen et al. 1984; Long et al. 2018; Phiri et al. 2019; Muleya et al. 2021). In Europe, the soils of Spain are typically adequately or highly supplied (Moreno Rodriguez et al. 2005), while they are low in Se in Finland (Hartikainen 2005) and in the UK (Broadley et al. 2006). The Se supply of soils in Eastern European countries is also low (Galić et al. 2023), so the estimated daily intake is usually below the recommended dietary allowance (Rayman et al. 2008).
The primary source of human and animal Se intake is food, so the lack of Se can be effectively compensated for by the biofortification of food crops. Agronomic biofortification involves the enrichment of a deficient element in the crop by applying it to the soil or growing medium, or in the form of a foliar spray (Galić et al. 2021). In addition to the quantity, the ratio of different Se species is also an important aspect of intake. Organic forms of Se have greater bioavailability and are more easily absorbed by human and animal organisms than inorganic forms, so their biological role is greater (Fairweather-Tait et al. 2010; Thiry et al. 2012). Inorganic Se forms (SeVI, selenite - SeIV) and organic Se forms (SeMet and high-Se-yeast) are all able to increase selenoenzyme (glutathione peroxidase) activity, but organic forms raise blood Se concentrations more effectively (Rayman et al. 2008). SeMet can be metabolized directly to reactive forms of Se or stored in body proteins (Schrauzer 2000). MetSeCys has been shown to have anti-tumour effects (Ip et al. 2002). Regarding overdoses, inorganic forms of Se are more acutely toxic than organic forms, and SeIV is more toxic than SeVI (Rayman et al. 2008). Organic forms may represent a greater danger in the long term due to accumulation, although some experiments have refuted this theory (Rayman 2004; Reid et al. 2004). Since the chemical form of Se in food crops largely determines the Se bioavailability in humans and animals, Se speciation is important for evaluating the effectiveness of biofortification (Kirby et al. 2008; Muleya et al. 2021).
Se is not an essential element for higher plants (White 2018). The Se species present in plant tissues are found in inorganic forms, typically as SeVI or in organic forms, as selenomethionine (SeMet) and methylselenocysteine (MetSeCys). One important aspect of biofortification is the Se tolerance of a given crop, that is, the amount of Se that can be taken up without having a toxic effect on the plant. SeVI is structurally similar to sulphate, so sulphate transporters in plant roots can absorb it from the soil (Terry et al. 2000; Hawkesford and Zhao 2007; Stroud et al. 2010). However, the majority of plants are unable to contain higher concentrations of Se, because Se is converted into the amino acids SeMet or selenocysteine (SeCys), which are incorporated into proteins (instead of methionine or cysteine) and inhibit their function (Gupta and Gupta 2017). This leads to toxic symptoms and, in more severe cases, to the destruction of the plant. At the same time, certain plants are able to accumulate Se in larger quantities without showing toxicity symptoms. Certain legumes species tend to accumulate more Se due to their high protein content (Poblaciones et al. 2014a), although the tolerance of some Se-accumulator Astragalus species is considerably greater than that of the sensitive species white clover (Trifolium repens L.) (Wu et al. 1994). Species in the Brassicaceae are able to incorporate Se into monomethylated amino acids, such as MetSeCys, which can accumulate without being incorporated into proteins (Terry et al. 2000; Ávila et al. 2014). They are also able to convert and store Se in the form of selenoglucosinolates (Wiesner-Reinhold et al. 2017). For example, cabbage may have a Se content as high as 100–1000 mg kg−1 in dry mass without showing symptoms of toxicity (Gupta and Gupta 2017). In the case of high Se content, one of the defence mechanisms of plants is the synthesis of volatile Se compounds (Zayed et al. 1998). Non-Se-accumulator plant species tend to produce dimethyl selenide, whereas hyperaccumulators primarily synthesise dimethyldiselenide (Pilon-Smits and Le Duc 2009). Even non-Se-accumulator species may have a high enough Se content to provide reasonable Se supplementation, as confirmed in the case of tomatoes (Pezzarossa et al. 2014), carrots (Smoleń et al. 2016; Oliveira et al. 2018) and green peas (Poblaciones and Rengel 2018).
Green peas, tomatoes, cabbage and carrots are widely grown and consumed almost all over the world; in 2020, the total production was 19.9, 186.8, 70.9 and 40.9 million tonnes on 2.53, 5.05, 2.41 and 1.13 million hectares, respectively (FAO 2022). Green peas are very rich in protein and essential amino acids, tomatoes and cabbage are consumed for their vitamins and antioxidant-rich phytochemicals, while carrots are high in fibre, carotenoids and sugars, and all these vegetables play an important role in human nutrition due to their mineral content (Nithiyanantham et al. 2012; Ali et al. 2021; Moreb et al. 2020; Smoleń and Sady 2009). Therefore, these crops may serve as good target plants for biofortification.
Se speciation (that is, the qualitative and quantitative distribution of different inorganic and organic forms of Se) may exhibit great diversity in different plant species (Fairweather-Tait et al. 2010). The ratio of organic to inorganic Se may also depend on the Se form with which the plant was supplemented. In leek, the inorganic form was present in a much higher ratio after supplementation with Na2SeO4 (55%) than with Na2SeO3 (21%), but the Na2SeO4 treatment resulted in a multiple Se concentration in the plant. In both treatments, MetSeCys and SeMet were the dominant organic Se forms (Lavu et al. 2012). The Se content of chickpea was also much higher after Na2SeO4 treatment and, depending on the inorganic Se treatment, 63–91% was converted into organic Se, the majority of which was SeMet (Poblaciones et al. 2014a). SeMet treatment resulted in SeMet dominance, and Na2SeO3 treatment in MetSeCys dominance in hydroponically grown lettuce (Kowalska et al. 2020). Muleya et al. (2021) reported that more than 90% of the Se applied as SeVI was transformed into organic form after plant uptake, and 74% of that was bioaccessible. The main organic Se form was SeMet in maize, groundnut and cowpea. SeMet had the largest ratio in durum wheat (Poblaciones et al. 2014b), rice grain (Yuan et al. 2023) and alfalfa (Kovács et al. 2023). In general, non-accumulator crop species, such as cereal crops, contain organic Se mainly in the form of SeMet, while in Se-accumulator species, like species in the Brassicaceae family, higher molecular mass Se species such as selenohomolanthionine, selenocystathionine and selenolanthionine are also found in higher ratios (Schiavon et al. 2020; Schiavon and Pilon-Smits 2017; Ogra et al. 2007; Both et al. 2020).
Quantitative changes in Se accumulation have been widely studied, but Se speciation studies have been performed to a much more limited extent. To the best of our knowledge, the analysis of Se species in ripe tomato fruit has not been reported so far.
The aim of the experiment was to study the effect of Se-enriched irrigation water and soil texture on the yield and element composition of green peas, tomatoes, cabbage and carrot plants (Ragályi et al. 2021, 2022). This work presents the results of quantitative analysis on the non-volatile Se species that are accumulated in the edible parts of the plants and can be quantified using an LC-ICP-MS instrumental setup, that is, with the help of commercially available standards. Based on the results of previous research, it was hypothesised that the SeVI content of individual plants would vary depending on the soil type, reflecting changes in their total Se content, and that the organic Se content would be significantly higher in cabbage than in the other vegetables.
Materials and methods
Experiment setup
The effect of irrigation with Se-enriched water was investigated in a greenhouse experiment on the research site of the Institute for Soil Sciences, Centre for Agricultural Research (Őrbottyán, Hungary). The test plants were grown in 10-litre pots with four holes (Ø 0.5 cm) in the bottom, containing a 1 cm layer of washed gravel (4–8 mm) covered with a fine synthetic fibre fabric on which 10 kg of homogenised topsoil was placed (Dobosy et al. 2020). Three topsoils (0–20 cm) with different textures were investigated: sand (Mollic Umbrisol, Arenic, from Őrbottyán, 47°40’N, 19°14’E), silty sand (Luvic Calcic Phaeozem from Gödöllő, 47°58’N, 19°38’E) and silt (Calcic Chernozem from Hatvan, 47°67’N, 19°64’E), the properties of which are shown in Table 1. The total number of pots was 36: 3 soils × 1 Se dose (500 µg Se L−1 actual concentration 561 ± 49 µg L−1) x 4 plant species x 3 replications. The entire experiment was designed with Se contents of 0, 100 and 500 µg L−1 in the irrigation water. Se speciation analyses, however, were only performed for samples given the highest quantity to make species detection more reliable, since the speciation methods used in this study had relevant limit of quantification (LOQ) values between 0.5 and 4.0 mg kg−1 in terms of dry mass. The test plants were tomato (Solanum lycopersicum, cv. Mano; ZKI) and cabbage (Brassica oleracea L. var. capitata cv. Zora; Réde) in 2018, and green pea (Pisum sativum L. var. Rajnai törpe) and carrot (Daucus carota L. var. sativus cv. Nantes-2) in 2019. Before the pot experiment, tomato, carrot and cabbage seeds were germinated and planted in propagation trays (1 seed per cell) filled with “VEGASCA Bio” (Florasca Hungary Ltd., Osli, Hungary) growing medium (a mixture of peat and grey cattle manure compost: organic matter > 50%; N > 0.3%; P2O5 > 0.1%; K2O > 0.1%; pH of 6.8). The seedlings were cultivated in a growth chamber for 22 days under controlled conditions (day/night temperatures and photoperiods of 26/18°C and 16/8 h, respectively, with a photon flux density of 500 µmol m−2 s−1 and relative humidity of 50–70%). The seedlings were irrigated with a total of 180 ml of tap water weekly, in 3 × 60 ml doses every Monday, Wednesday and Friday. After a 6-day acclimatisation period in the greenhouse, the soil-free seedlings were transplanted into the experimental pots: 1 seedling per pot for cabbage and tomato and 3 seedlings per pot for carrot. Germinated green pea seeds were sown directly (3 seeds per pot). In the case of both carrots and green peas, 3 plants per pot were grown during the whole vegetation period.
The plants were irrigated with an automated system using individual drip stakes placed in each pot. During the growing period, each pot was watered weekly with Hoagland solution (200 ml per pot). For the first three weeks after planting, tap water was applied without Se treatment. Irrigation with Se solution (Na2SeO4 diluted with tap water) started three weeks after planting. The Se content of the irrigation water averaged 0.287 ± 0.044 µg Se L−1. The solution in the tank was regularly monitored to ensure a constant Se concentration. The tap water used to prepare the Se solution was stored in 0.5 m3 tanks (separate tanks for each irrigation solution) before application to reduce the chlorine concentration. The volume of irrigation water was adjusted to the water requirements of the different plant species. Soil moisture content was monitored at a depth of 10 cm every hour (Decagon EC-5, ICT International, Armidale, Australia). The irrigation system delivered the pre-set amount of water at 7 a.m. each day. The details of growing periods, plant protection and irrigation are shown in Supplemental Materials (Table S1).
The experimental area received natural light. The greenhouse climate data (mean day and night temperature, air humidity and photon flux density of photosynthetically active radiation) were continuously monitored during the growing period (Table 2). Pesticides and fungicides were applied as necessary.
Chemical analysis
The untreated composite soil samples used for the experiment were collected, dried, sieved through a 2 mm mesh and analysed. The soil pH was measured in a 1:2.5 soil:water suspension after mixing for 12 h. The following analytical tests were performed: total N content with the Kjeldahl method (ISO 11261:1995), organic matter (OM) content with the modified Walkley–Black method (FAO 2020), CaCO3 content with the Scheibler gas-volumetric method (ISO 10693, 1997), and cation exchange capacity (CEC) with the modified method of Mehlich (ISO 13536:1995). The total Se concentrations were analysed after microwave-assisted digestion with aqua regia in a Teflon digestion vessel (ISO 12914:2012). The plant-available P and K fractions were measured after extraction with ammonium acetate-lactate (AL-P and AL-K) (Egnér et al. 1960), and extractable Se (LE-Se) was measured using 0.5 M NH4-acetate + 0.02 M EDTA extract (Lakanen and Erviö 1971).
After harvest, the plants were cleaned with deionized water, then the roots, shoots (aboveground vegetative parts) and fruit were separated, and the fresh weight of the plant parts was measured. The cabbage leaf samples were dried at 40 °C for two days in a laboratory oven, while tomato fruit, green pea seed and carrot root samples were milled and freeze-dried at -70 °C in Christ Alpha 1 (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) equipment (200 Pa for 72 h) followed by determination of dry mass. All the samples were homogenised in a blending machine equipped with plastic housing and a stainless-steel blade. The dried, homogenised samples were kept in centrifuge tubes in a dark room at room temperature until analysis.
For total Se determination, the dried, homogenised samples were mineralised in a microwave-assisted acid digestion system (TopWave, Analytik Jena, Germany). Dried plant samples (400–500 mg) were digested in a mixture of 7 cm3 67% HNO3 and 3 cm3 30% H2O2. After digestion, the internal standards were added to the solutions, and the volume was made up to 15 cm3 with deionised water. The concentrations of Se and other elements in the soil and plant samples were measured with an inductively coupled plasma mass spectrometer (ICP-MS, PlasmaQuant Elite, Analytik Jena, Germany) with a determination precision of 1–3% relative standard deviation (RSD) for Se. The total Se content and biomass production of plants have been published elsewhere (Ragályi et al. 2021, 2022).
Sample preparation for speciation analysis using strong anion exchange (SAX) HPLC-ICP-MS
For the sample preparation and quantification of SeMet, MetSeCys and SeVI, the methods published by Shao et al. (2014) and in the Annex of the Commission Implementing Regulation (EU 2020/2117) were taken and adapted. In short: dried and homogenised samples were further milled with a MM 200 mixer mill (Retsch GmbH, Haan, Germany). About 0.25 or 0.50 g of milled samples were weighed into centrifuge tubes for extraction. For the extraction of water-soluble compounds, 10 ml deionised water was added to the samples, which were sonicated in an ultrasonic bath for 15 min at room temperature. They were then centrifuged at 8000×g for 10 min. The supernatants were removed and filtered using a 0.22 μm pore size hydrophilic PTFE disposable syringe filter. In the case of peas, a starch deposit was observed in the clear supernatant, so filtration was done almost immediately before injection. The solid residues were enzymatically digested with Pronase E (from Streptomyces griseus, 4,000,000 PU g−1, Merck-Sigma, Darmstadt, Germany). About 40 mg enzyme per sample was dissolved in 9 ml 100 mM TRIS buffer (pH = 9, adjusted with 1 M HCl) and mixed with the plant residues. The closed centrifuge tubes were shaken in a water bath at 37 °C overnight. An additional 40 mg Pronase enzyme per sample was dissolved in 1 ml TRIS buffer and added to the samples on the following day. After shaking for a further 6 h at 37 °C, the samples were centrifuged at 8000×g for 10 min. The supernatants were removed and filtered through a 0.22 μm pore size hydrophilic PTFE disposable syringe filter. Extracted samples were stored at + 4 °C and analysed within 2 days. Before analysis, dithiothreitol (Merck-Sigma) was added to reach a 0.01% m/m concentration in the samples to prevent the formation of oxidised SeMet. Enzymatic extraction efficiency and method accuracy were regularly checked and corrected for recovery with the help of the Se-enriched yeast (SELM-1) reference material (LGC Standards Ltd., Teddington, UK). One plant was sampled from each of the three replicates, and one extraction was carried out on each sample. In order to estimate the LOQ, three parallel injections were done from the 10, 20, 50 and 100 µg L−1 standard solutions of the selenocompounds. Regarding the precision of the instrumental technique, RSD was calculated for the peak areas and the results were checked against the acceptance level (RSD ≤ 10%).
Se speciation by HPLC-ICP-MS
Species in water extract and enzymatic extract were separated by SAX chromatography and detected using ICP-MS. A Waters Alliance 2695 HPLC instrument (Waters Co., Milford, MA, USA) was coupled to an X-Series II ICP-MS (Thermo Scientific, Bremen, Germany). The injection volume was 10, 20, or 40 µl depending on the analyte concentrations. A Hamilton PRP-X100 (250 × 4.1 mm, 10 μm) SAX column (Hamilton Company, Reno, NV, USA) was used at a temperature of 25 °C. Gradient elution was done with ammonium acetate (pH = 6.5, eluent A: 10 mM, eluent B: 250 mM) at a 2.0 ml min−1 flow rate. The programme was as follows: 0–1 min: 0% B, 1–20 min: up to 100% B, 20–29 min: 100% B, 29–31 min: down to 0% B, 31–37 min: 0% B. If MetSeCys was found to exceed the LOQ in the samples, it was quantified in separate injections with a modified LC gradient (0–1 min: 0% B, 1–2 min: up to 5% B, 2–3 min: up to 100% B, 3–10 min: 100% B, 10–11 min: down to 0% B, 11–18 min: 0% B).
The ICP-MS parameters were as follows: forward power 1400 W, plasma gas flow rate 14.0 l min−1, nebuliser gas flow rate 0.86 l min−1, auxiliary gas flow rate 0.88 l min−1, pole bias − 16.0 V, hexapole bias − 10.0 V, dwell time 100 ms. H2–He (in 7:93%) collision cell gas was applied with a 6.0 ml min−1 flow rate. A concentric nebuliser was used with a Peltier-cooled conical spray chamber (2 °C). The detected ions were 78Se and 80Se, but 78Se was used for quantification. Standard addition was applied for the quantification of MetSeCys, SeMet and SeVI (Merck-Sigma Group) as shown in Supplemental Materials, Figs. S1–S3. Concentrations are expressed in dry mass.
Post column isotope dilution analysis
A different method, post column isotope dilution analysis, was applied for the quantification of species in the enzymatic digestion of green pea samples due to high matrix interference.
An isotopically enriched spike solution was diluted with deionized water from a 10 mg 82Se L−1 stock solution (Inorganic Ventures, Christiansburg, VA, USA) to 20 ng g−1. The spike solution was continuously dispensed into the effluent via a T-piece using a syringe pump at 0.1 g min−1 and introduced into the ICP-MS. The HPLC and ICP-MS parameters were the same as those described for the HPLC(SAX)-ICP-MS analysis. The monitored isotopes were m/z 76, 77, 78, 80, 81, 82 and 83. The intensity of hydride formation (1.5%) and the mass bias factor (-4.9% per mass unit) were determined based on the calculations of Hinojosa Reyes et al. (2003a). Intensity values of m/z 77, 78, 80 and 82 were corrected for hydride formation and mass bias. The mass flow of Se in the effluent was determined using the natural isotope ratio of Se and the certified isotope ratios in the spike solution, corrected with a blank injection. The amount of Se species was quantified by summarising the mass flow in the retention window of the individual species in Microsoft Excel, taking into account the dwell times and the detector dead time. The concentration was determined from the amount of Se species and the injection volume (Hinojosa Reyes et al. 2003b; Ouerdane et al. 2020).
Elemental Se analysis
Elemental Se was measured from tomato and green pea samples. After water extraction and enzymatic digestion, plant residues were extracted with Na2SO3 extractant based on the method of Both et al. (2018) with minor modifications. Five millilitres of 1.0 M sodium sulphite (Merck-Sigma Group) was added to the residual plant materials. The samples were vortexed and shaken in a water bath at 37 °C overnight, and then centrifuged at 8000×g for 10 min. After removing the supernatants, 4 ml of additional deionised water was pipetted to the residues. The samples were vortexed for one minute and were again centrifuged at 8000×g for 10 min. The supernatants were merged and filled up to 10 ml with deionised water. Five ml of the Na2SO3 extracts was pipetted into PTFE vessels, then mixed with 7 ml nitric acid (65% w/w) and 1 ml hydrogen peroxide (30% w/w) from Scharlab S.L. (Sentmenat, Spain). The digestion was performed in a microwave-assisted digestion system (Milestone Start D, Sorisole, Italy) at 180 °C. Digested samples were filled up to 25 ml with deionised water. Before analysis, a 25 times dilution was applied, and an Rh internal standard was added. ICP-MS (X-Series II, Thermo Scientific, Bremen, Germany) was used for the quantification with the standard addition method. Instrument parameters were the same as those given for Se speciation with HPLC(SAX)-ICP-MS.
Residual Se content in plant residues and in extracts
After water extraction, enzymatic digestion and sodium sulphite extraction, the solid plant residues of tomato and green pea were washed into PTFE vessels with 7 ml nitric acid (65% w/w), after which 1 ml hydrogen peroxide (30% w/w) was added. The digestion and analysis parameters were the same as those used for elemental Se analysis.
The water extract and the enzymatic extract of green pea samples were also digested to determine the total Se content. The extractions were repeated and 5.0 ml of the homogenised extracts was pipetted into PTFE vessels. The pipetted water extract contained a proportionate amount of the starch deposit that appeared shortly after the extraction. The digestion and analysis parameters were the same as those used for elemental Se analysis.
Statistical analysis
The data were analysed for treatment effects using factorial analysis of variance and Tukey’s post-hoc test. In the case of the Se species contents measured in fresh edible parts, the assumption of homogeneity of variances was not met based on Levene’s test, so the Kruskal-Wallis test was used. The variances were calculated for soil types and vegetable species. Significant differences between the treatments were calculated at the p < 0.05 level. Statistica v.13 (StatSoft Inc.) software was used for all the statistical evaluations. Data visualisation was implemented with MS Excel 2016 and R statistical software (R Core Team 2022) using the ggplot2 package (Wickham 2016).
Results
Total Se content
The soil types used in the present experiment affected the total Se concentration measured after HNO3-H2O2 digestion. The Se concentration of cabbage leaf (150 ± 6 mg kg−1) and green pea seed (37.1 ± 6.9 mg kg−1) on sandy soil was significantly higher than on silty sand (116 ± 5 and 29.0 ± 3.9 mg kg−1, respectively) and silt soils (119 ± 9 and 24.0 ± 1.6 mg kg−1, respectively). The Se concentration of carrot root on silt soil (25.5 ± 1.8 mg kg−1) was significantly higher than that measured on sandy soil (17.6 ± 3.2 mg kg−1), whereas its concentration on silty sand (22.5 ± 3.9 mg kg−1) was not significantly different from that measured on the other two soil types. The Se concentration of tomato fruit was significantly higher on silty sand and silt soils (23.5 ± 1.0 and 24.8 ± 0.5 mg kg−1, respectively) than on sand (21.6 ± 1.4 mg kg−1) (Ragályi et al. 2021, 2022).
Concentrations of SeVI, MetSeCys and SeMet in water extract and enzymatic extract
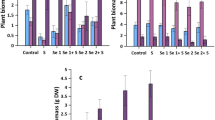
The concentrations of water-extractable and enzymatic digestion-extractable Se species of vegetables are shown in Fig. 1. The soil type did not cause a significant difference for any of the Se species investigated. SeVI was found in both water extract and enzymatic extract in cabbage and carrot, but only in water extract in tomato and pea. Water-extractable SeVI was found in the highest concentration in cabbage (between 46.0 and 66.9 mg kg−1). After enzymatic digestion, additional SeVI was extracted (between 7.35 and 11.8 mg kg−1). In the case of carrot roots, the water-extractable SeVI was 12.0–17.0 mg kg−1, and after enzymatic digestion, additional SeVI was detected, but only at a much lower concentration (1.67–2.57 mg kg−1). SeVI was found in the water extracts of tomatoes in the range of 2.19–3.44 mg kg−1, while in pea seeds it reached 1.86–4.12 mg kg−1 in the water-soluble fraction.
Water-extractable (WE) and enzymatic digestion-extractable (ED) SeVI, MetSeCys and SeMet species concentrations in vegetables grown on different soil types. In the missing cases, the specific Se species in the given extractant was below the LOQ. LOQ values were: WE-SeMet: 0.5 mg kg−1, WE-MetSeCys: 1.0 mg kg−1 for cabbage; WE-SeMet: 0.5 mg kg−1, WE-MetSeCys: 1.0 mg kg−1, ED-MetSeCys: 2.0 mg kg−1 for carrot; WE-SeMet: 0.5 mg kg−1, WE-MetSeCys: 1.0 mg kg−1, ED-SeVI: 0.8 mg kg−1, ED-MetSeCys: 4.0 mg kg−1 for tomato; and WE-SeMet: 0.5 mg kg−1, WE-MetSeCys: 1.0 mg kg−1, ED-SeVI: 0.3 mg kg−1, ED-MetSeCys: 1.5 mg kg−1 for pea. Error bars represent the standard deviation of the means (n = 3). n.s.: no significant difference for different soil types
MetSeCys only exceeded LOQ in the enzymatic digestion-extractable fraction of cabbage, with a concentration of between 2.63 and 2.87 mg kg−1. Figure 2 represents a typical chromatogram of a cabbage leaf extract after enzymatic digestion. MetSeCys is only retained to a low extent in SAX, so its presence must be identified by spiking. When a sample was spiked, the presence of MetSeCys was confirmed. The compound was then analysed by standard addition (Fig. 2b). MetSeCys also appeared in carrot root, tomato fruit and green pea seed, but was below LOQ. Further chromatograms for the water and enzymatic digestion extracts of the vegetables, with short descriptions, can be found in Supplemental Materials, Figs. S4–S8.
SeMet could not be detected in water extract, but exceeded the LOQ in the enzymatic digestion-extractable fraction of all four vegetables. Its concentration was 4.94–6.13 mg kg−1 in cabbage, 1.23–1.50 mg kg−1 in carrot, 7.10–8.35 mg kg−1 in tomato and 4.13–4.89 mg kg−1 in pea (Fig. 1).
The enzymatic extracts of pea were analysed using post column isotope dilution analysis (HPLC-(IDA)-ICP-MS) instead of standard addition due to matrix (probably starch-based) interferences. A typical chromatogram for enzymatically digested pea is presented in Fig. 3a, while the mass flow of Se is illustrated in Fig. 3b for the same injection.
Concentrations of elemental and residual Se
The combined concentrations of water-soluble and enzymatic digestion-extractable Se species in tomato fruits were 9.54–11.04 mg kg−1, about three quarters (67.6–77.3%) of which were SeMet and the remainder SeVI (22.7–32.4%). However, 55.1–55.9% of the total Se content was not detectable. Also, in peas, the concentration of quantified species was 5.98–10.07 mg kg−1, which was only 25.0-26.9% of the total Se content determined in samples digested with HNO3-H2O2. Therefore, the analyses were continued, and elemental Se, an often dismissed form, was quantified in plant residues after water extraction and enzymatic digestion. The 1.0 M Na2SO3-extractable Se was identified as elemental Se. A relatively high amount (5.37–6.17 mg kg−1) of elemental Se was found in tomato fruit, whereas in pea it was much lower, between 1.33 and 2.30 mg kg−1. However, in the case of pea, there was a significant difference between the soil types. The elemental Se concentration of pea grown on sand soil was significantly higher than on silty sand and silt. The concentration of residual Se was between 5.73 and 8.67 mg kg−1 in tomato, and between 0.433 and 0.733 mg kg−1 in pea (Fig. 4).
Elemental and residual Se species concentrations of tomato fruit and pea grown on different soil types. Error bars represent the standard deviation of the means (n = 3). Different lower case letters indicate significant differences for different soil types. n.s.: no significant difference between soil types
Ratios of Se species and concentration in fresh vegetables
After water extraction and enzymatic digestion, the combined concentration of soluble Se species in cabbage was 61.5–87.6 mg kg−1, depending on the soil type. This concentration range accounted for 51.6–63.7% of the total Se, whereas other fractions, which were not soluble using the methods applied, accounted for 36.3–48.4% (Fig. 5). SeVI made up the largest part with 45.0-56.5%, while the organic SeMet and MetSeCys species together made up 5.98–7.21%.
In carrot, the combined concentration of soluble Se species in the water extract and enzymatic extract was 13.7–19.5 mg kg−1, which was about three-quarters (77.9–84.0%) of the total Se content. The overwhelming majority, about 90.2–92.9%, of the extractable Se species was inorganic, namely SeVI, while SeMet accounted for the rest.
After the identification of the elemental and residual Se fractions in tomato, 94.5–100% recovery was reached, which was the highest for any of the vegetables studied. The proportions of SeMet and residual Se were in a similar range (28.0-34.1% and 26.6–34.1%, respectively, of the total Se content), and a relatively high proportion (23.6–24.9%) was found in elemental Se form in tomato fruit, while the SeVI ratio was lower (10.1–13.5%).
In peas, the mass balance was still not complete after analysing the water extract, enzymatic extract, elemental and residual fractions. The combined species concentrations obtained were only 32.4–34.0% of the total Se. Among the species identified, SeMet was dominant with 14.7–17.2% of total Se, followed by SeVI with 7.75-11.0%, elemental Se with 4.83–6.20% and residual Se with 1.73–1.98% (Fig. 5). It should be noted that after the centrifugation of the water extract, a starch deposit was observed in the clear supernatant. Speciation analysis was done on a filtered extract prepared almost immediately before injection. In contrast, digestion was performed on a homogenised portion of the supernatant (containing the deposit).
The soil type had no significant effect on the amount of Se species found in 100 g fresh weight of the edible part of the plant for any of the species, so the data are presented as the average of the three soil types (Fig. 6). Differences between the vegetables, on the other hand, were significant. MetSeCys was only present in measurable amounts in cabbage. SeMet was found in the highest amount in fresh green peas, and this was significantly higher than in tomato fruit or carrot root. The second highest amount was found in cabbage, which was significantly higher than carrots, but not significantly different from tomatoes or peas. The amount of SeMet in tomatoes was similar to that in cabbage. The SeVI content in 100 g fresh edible part of cabbage was significantly higher than that in tomatoes and peas. The second highest SeVI content was found in carrots, which was significantly higher than in tomatoes.
Discussion
For several reasons, it is rather difficult to interpret the results of Se speciation analyses in the context of results from other experiments. On the one hand, there are relatively few reports of Se speciation studies, and even fewer discuss the analysis of the vegetables examined in this experiment. On the other hand, even if the same plant species is examined, several other factors also have an influence, for example, the form of Se (SeIV or SeVI) used for biofortification (Poblaciones et al. 2014a; Kápolna et al. 2009). The level of Se enrichment, that is, the Se concentration achieved in the biofortified plant, also has an influence on the ratio of Se species (Poblaciones et al. 2014a; Garousi et al. 2017). The extraction technique, extraction agent, the amount of enzyme (Mazej et al. 2006; Luo et al. 2022) and the incubation time (Cuderman et al. 2008) also largely determine the results (Montes-Bayón et al. 2006). Sánchez-Rodas et al. (2016) found considerable differences between the devices used for analysis. Different cultivation conditions (soil vs. hydroponics, root uptake vs. leaf spraying) may also lead to different results (Maneetong et al. 2013).
In the present experiment, the water- and enzyme-soluble Se species accounted for 57.6%, 81.4%, 44.4% and 26.0% of all Se in the case of cabbage, carrots, tomatoes and green peas, respectively (not including the elemental and residual fractions of tomatoes and peas). A similar result was obtained for cabbage by Mechora et al. (2012), with 60% solubility after digestion with the enzyme protease XIV. Cuderman et al. (2008) found that soluble Se species accounted for 51–68% of the total Se content in potatoes. The higher proportion of the insoluble form might be the result of a detoxification mechanism, such as binding to phenolic substances (Vogrinčič et al. 2009). Water extraction extracted 44.2, 65.4, 11.8 and 10.7% of total Se from cabbage, carrots, tomatoes and green peas, respectively. Enzymatic digestion was therefore the most efficient in the case of tomatoes, where it was able to extract an additional 32.6%, while in the case of the other three plants, it only contributed 13.4–16.0% to the extraction efficiency. Stadlober et al. (2001) reported a greater increase: in the cereals investigated, water extracts could only identify 3–9% of the total Se content, while with enzymatic digestion this ratio increased to 80–95% due to the extraction of selenospecies from the protein matrix. In the case of peas, the proportion of soluble species after water extraction and enzymatic digestion was the lowest. One possible explanation would be the loss of late eluting species, but in the present case, an inherently long separation lasting 36 min was chosen. Compared to methods involving shorter gradients such as those described by Kowalska et al. (2020) and Luo et al. (2022), this gradient would have recovered late eluting analytes, even if long gradients might cause flattening of the peaks. Another possible explanation is the high starch content of the sample. The missing amount of Se could have been absorbed into the starch deposit and/or irreversibly retained (precipitated) on the column. Indeed, the complementary application of amylase enzymes would not generally provide adequate results because of either low recovery (< 35% for SeMet, according to Cuderman et al. 2008) or their inherently high Se content, which biases the accuracy of the results (Cuderman and Stibilj 2009).
In the case of cabbage, SeVI was dominant both in water and enzymatic extracts (Fig. 1). Similar results were obtained by Slekovec and Goessler (2005) after treatment with 20 mg Se m−2 in the form of Na-selenate, which increased the Se content of cabbage leaves to 12 mg kg−1. The 9:1 methanol:water extraction resulted in an extraction efficiency of only 20%, after which approximately 90% of the total Se content was found in SeVI form. In contrast, Mechora et al. (2012) detected an increase in Se concentration to 4.77 mg kg–1 in Na-selenate treated cabbage leaves, in which SeMet represented 13% of total Se, whereas SeVI and SeIV represented only 3.0 and 2.9%, respectively, after protease XIV extraction. The higher average SeVI rate of 88% in the present experiment was possibly due to the much higher (128 mg Se kg−1) Se content in cabbage leaf as a result of the treatment (Ragályi et al. 2021). In the current experiment, MetSeCys was practically only detected in cabbage, confirming that this Se species is typical of the Brassicaceae family. The presence of MetSeCys was confirmed after spiking, which is a very important step in species assignment when only element- (that is, Se-) specific detection is addressed, as retention times might shift because of matrix effects and column load (Kápolna et al. 2007). The need for enzymatic sample preparation to extract non-proteinaceous, that is, non-protein-bound methylated Se species, was previously reported for selenised bean and mushroom samples (Shao et al. 2014; Egressy-Molnár et al. 2016) and was proved here for cabbage as well; indeed, the decomposition of sample matrices is important to achieve adequate species recovery even for theoretically water-soluble compounds. Although SeVI treatment mostly increases the Se content of the plant more effectively than Se IV treatment, since SeVI is quickly translocated to the above-ground organs, most of the Se taken up in this way tends to remain in SeVI form, and it may happen that only a small proportion is converted into SeMet or other organic forms (Zayed et al. 1998). Generally, the relatively high SeVI concentration in plants can be explained by the fact that it is chemically similar to SO42−, so the sulphate transporter can mediate the uptake of both anions through the root plasma membrane. The reduction of SeVI in the plant by the enzyme ATP sulphurylase is the first step in the conversion to organic Se, but this is energy-demanding, and ATP sulphurylase is a rate-limiting factor (Pilon-Smits et al. 1999; Terry et al. 2000). Thus, in cases of high SeVI application, this species may be taken up by the plant in large amounts, but little of it may be converted, so SeVI accumulates.
SeVI was also dominant in carrots, particularly in the case of water extraction, while enzymatic extraction released additional SeVI and an almost equal amount of SeMet (Fig. 1). Different results were obtained by Bañuelos et al. (2015), who found no significant increase in the recovery percentage of SeMet after the enzymatic digestion of carrot roots, in which the total Se content was 2.5 mg kg−1. Kápolna et al. (2009) applied SeVI to carrots in the form of a leaf spray. After digestion with proteolytic enzymes, Se mainly occurred in the form of SeMet and γ-glutamyl-selenomethyl-selenocysteine, and the SeVI concentration was only half that of SeMet. The different results may be due to the different mode of application (leaf spraying), since the reduction and conversion of SeVI into organic forms occur inside the chloroplasts in the shoots.
Although the Se biofortification of tomatoes has been investigated, few studies have been made on the Se speciation of ripe tomatoes. Asher et al. (1977) studied the transport of Se across the tomato root, Schiavon et al. (2013) reported the effect of Se on the chemical composition of tomato, and Brummell et al. (2011) investigated the effect of Se treatment on the MetSeCys production of transgenic tomatoes. Based on the results, the Se biofortification of tomatoes appears to have a positive effect, since in the present work, when four vegetable crops were treated under the same conditions, peas and tomatoes contained the highest ratios of organic Se (Fig. 5).
The water extract of green peas only contained SeVI, but the amount of SeMet extracted by enzymatic digestion was even higher (Fig. 1). A similar result was obtained by Garousi et al. (2017), who identified SeMet as the main component in green pea seed after enzymatic hydrolysis. Smrkolj et al. (2006) investigated plants grown from Se-enriched pea seeds. SeMet was the major Se species, but a higher SeMet ratio was reached using the lower Se treatment in the leaves and the higher Se treatment in the seeds. The composition of Se species was found to vary not only with the plant parts but also with plant maturity. In contrast, Yoshida et al. (2005) found the major part of Se in the form of SeVI in the pods and leaves of SeVI-treated green peas, while SeMet was only present in a minor proportion. The difference may be due to the fact that pea had a rather high total Se content of 68 mg kg−1, while in the present experiment it was only 30 mg kg−1 (Ragályi et al. 2022). Poblaciones et al. (2014a) applied SeVI as foliar spray in a field experiment. Interestingly, as the Se treatment level increased, the proportion of SeVI decreased and the proportion of SeMet increased in the chickpea test plant. About 84–91% of Se was found in the form of SeMet, but at the same time, the occurrence of SeVI (4–7%) was also significant. In the Se speciation analysis, the proportion of Se recovered was 57–67% of the total Se content.
The results of the present experiment show that the accumulation of SeVI is typical in the vegetative organs (cabbage leaf and carrot root) while that of SeMet is mainly observed in the reproductive organs (tomato fruit and green pea seed) (Fig. 5). This may be related to the transport and transformation of Se in plants. As mentioned earlier, the absorption of SeVI and its translocation in the plant takes place with the help of sulphate transporters, so the vegetative plant organs are able to accumulate SeVI. By following the S assimilation pathway, the SeVI can be further transformed into Se-amino acids, which predominantly takes place in the plastids in the leaves and result in the formation of SeMet (Pilon-Smits et al. 1999). Sulphur-containing methionine derivates can be translocated through the phloem to the reproductive organs, where they are released for development and storage (Bourgis et al. 1999), as was also demonstrated in green peas (Tan et al. 2010). Assuming that the pathways of secondary Se metabolites in plants are analogous to their sulphur counterparts (White 2018), SeMet may also have followed this pathway in the present experiment, possibly explaining their higher ratio in the reproductive organs of tomatoes and green peas.
In 100 g of fresh edible parts, the largest amount of detectable Se species was found in cabbage leaves, but the highest amount of organic Se, in the form of SeMet, was found in green peas, a significantly higher amount than in carrots or tomatoes (Fig. 6). Tomatoes, on the other hand, contained an extremely high, nearly three-fold ratio of organic Se compared to inorganic Se. When evaluating the effectiveness of Se species in terms of increasing Se levels in humans or animals, several aspects must be taken into account. In general, selenium is absorbed efficiently, but organic Se forms are more efficient in increasing the Se content of the human or animal body than inorganic forms (Steen et al. 2008). The uptake of inorganic forms also proved effective, especially in the case of SeVI, since 50 and 91% of SeIV and SeVI, respectively, was absorbed from a Se-fortified milk-based formula (Dael et al. 2001). At the same time, the organic forms of selenium, such as SeMet, are retained better in tissues, because they are incorporated nonspecifically into proteins, making them instrumental in Se storage (Huerta et al. 2004). MetSeCys has been investigated more widely for its inhibition of carcinogenesis (Whanger 2002), but this species has to be metabolised to be used for selenoprotein formation or excretion, so in the case of overload, this compound and its potential metabolites might cause cytotoxicity (Marschall et al. 2016). According to Fairweather-Tait et al. (2010), it is not possible to quantitatively evaluate the effectiveness of different forms of Se in terms of bioavailability and retention, because of the complexity of the foods containing them. The typical consumption method of the biofortified food might also be an important aspect. The cooking or salting of fish did not affect Se absorption or retention (Fox et al. 2004), and in wheat, only a small reduction in SeMet content was detected after processing and bread making (Hart et al. 2011). However, heat processing involving pressure-cooking or microwave cooking significantly reduced the bioaccessibility of Se in cereals (14.8–55% and 8–51%, respectively), pulses (7.6–35.1% and < 43%, respectively) and green leafy vegetables (< 64% and < 52%, respectively) (Khanam and Platel 2016). These results justify the biofortification of tomatoes, which are widely consumed raw.
Elemental Se has different bioavailability compared to other inorganic forms (Kojouri et al. 2012), while its toxicity differs not only from that of other inorganic Se forms, but also from that of SeMet (Wang et al. 2007). The occurrence of elemental Se in living organisms can be linked to either bacterial activity (Tomei et al. 1995; Oremland et al. 1989; Kessi et al. 1999) or possible Se detoxification (Both et al. 2018; Pilon-Smits 2019), which may take place via several not yet elucidated pathways. According to Kim et al. (2003), who detected elemental Se in barley grain, SeIV is probably reduced by thioredoxin to selenide, which in turn is autoxidised to elemental Se. Van Hoewyk et al. (2005 and 2008) found that NifS-like proteins with selenocysteine lyase activity catalyse the breakdown of SeCys into alanine and elemental Se, as demonstrated in Arabidopsis thaliana. Lindblom et al. (2013) reported that elemental Se was the second most abundant Se form in field-collected roots of Astragalus bisulcatus and Stanleya pinnata, which was attributed to the microbial activity of endosymbionts including nitrogen-fixing bacteria and endophytic fungi or bacteria. Based on the studies of Valdez Barillas et al. (2012) with Astragalus bisulcatus, elemental Se accounted for 11–31% of the Se species identified in the root, 34% in the stem, 7% in the flower and 20% in the cotyledons of the infected seed embryo. Both et al. (2018) found that in the Se hyperaccumulator plant Cardamine violifolia (Brassicaceae), inorganic Se was mostly present in the form of elemental Se. To the best of our knowledge, elemental Se has not yet been reported in the edible parts of vegetables biofortified with SeVI.
In order to evaluate the results and the success of biofortification, it is important to note that the fresh biomass of cabbage head and tomato fruit decreased non-significantly by 12 and 9%, respectively, in the Se treatment compared to the control (which were not subject to Se speciation analysis), but the dry matter content increased significantly in both plants, so dry biomass production changed only slightly. The yield of green peas exhibited practically no change, while the fresh biomass of carrot root decreased non-significantly by 10% and the dry biomass significantly by 20% (Ragályi et al. 2021, 2022). Based on these data, carrots are less able to tolerate higher Se content, while the cultivation of green peas is very promising on soils with higher Se supplies.
Conclusions
This work aimed to present and evaluate the Se species composition of vegetable plants widely consumed by the human population and treated under identical conditions. As regards the total amount of Se species recovered, cabbage contained by far the most Se due to its inherent ability to accumulate this element as a member of the Brassicaceae family, so cabbage biofortification can be considered the most effective. At the same time, the concentration of the more bioavailable SeMet was the highest in the dry matter of tomatoes, though due to the higher dry matter content, green peas contained the highest amount of SeMet in 100 g of fresh edible plant parts, so the biotransformation was the most effective for these two vegetables. The Se treatment only caused a significant decrease in biomass in the case of carrots, so the cultivation of cabbage, tomatoes and green peas could be well suited to soils with a high Se content. The different results obtained for Se species draw attention to the fact that the metabolism of Se differs in different plant species. Further research is needed to explore the effect of different doses, forms and sources of Se on the Se speciation in different vegetables in order to make the biofortification process more effective and controlled.
Data availability
The datasets analysed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- LOQ:
-
Limit of quantification
- MetSeCys:
-
Methylselenocysteine
- RSD:
-
Relative standard deviation
- SAX:
-
Strong anion exchange
- Se:
-
Selenium
- SeCys:
-
Selenocysteine
- SeMet:
-
Selenomethionine
- SeIV:
-
Selenite
- SeVI:
-
Selenate
References
Ali MY, Sina AAI, Khandker SS, Neesa L, Tanvir EM, Kabir A, Khalil MI, Gan SH (2021) Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease. Rev Foods 10:45. https://doi.org/10.3390/foods10010045
Asher CJ, Butler GW, Peterson PJ (1977) Selenium transport in root systems of tomato. J Exp Bot 28:279–291
Ávila FW, Yang Y, Faquin V, Ramos SJ, Guilherme LRG, Thannhauser TW, Li L (2014) Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem 165:578–586. https://doi.org/10.1016/j.foodchem.2014.05.134
Bañuelos GS, Arroyo I, Pickering IJ, Yang SI, Freeman JL (2015) Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem 166:603–608. https://doi.org/10.1016/j.foodchem.2014.06.071
Both EB, Shao S, Xiang J, Jókai Z, Yin H, Liu Y, Magyar A, Dernovics M (2018) Selenolanthionine is the major water-soluble selenium compound in the selenium tolerant plant Cardamine violifolia. BBA - General Subjects 1862:2354–2362. https://doi.org/10.1016/j.bbagen.2018.01.006
Both EB, Stonehouse GC, Lima LW, Fakra SC, Aguirre B, Wangeline AL, Xiang J, Yin H, Jókai Z, Soós Á, Dernovics M, Pilon-Smits EAH (2020) Selenium tolerance, accumulation, localization and speciation in a Cardamine hyperaccumulator and a non-hyperaccumulator. Sci Total Environ 703:135041. https://doi.org/10.1016/j.scitotenv.2019.135041
Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen TL, Gage DA, Hanson AD (1999) S-Methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell 11:1485–1498. https://doi.org/10.1105/tpc.11.8.1485
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65:169–181. https://doi.org/10.1079/PNS2006490
Brummell DA, Watson LM, Pathirana R, Joyce NI, West PJ, Hunter DA, McKenzie MJ (2011) Biofortification of tomato (Solanum lycopersicum) fruit with the anticancer compound methylselenocysteine using a selenocysteine methyltransferase from a selenium hyperaccumulator. J Agric Food Chem 59:10987–10994. https://doi.org/10.1021/jf202583f
Cuderman P, Kreft I, Germ M, Kovačevič M, Stibilj V (2008) Selenium species in selenium-enriched and drought-exposed potatoes. J Agric Food Chem 56:9114–9120. https://doi.org/10.1021/jf8014969
Cuderman P, Stibilj V (2009) How critical is the use of commercially available enzymes for selenium speciation? Anal Bioanal Chem 393:1007–1013. https://doi.org/10.1007/s00216-008-2495-9
Dael P, Davidsson L, Muñoz-Box R, Fay L, Barclay D (2001) Selenium absorption and retention from a selenite- or selenate-fortified milk-based formula in men measured by a stable-isotope technique. Br J Nutr 85:157–163. https://doi.org/10.1079/BJN2000227
Dobosy P, Endrédi A, Sandil S, Vetési V, Rékási M, Takács T, Záray G (2020) Biofortification of potato and carrot with iodine by applying different soils and irrigation with iodine-containing water. Front Plant Sci 11:593047. https://doi.org/10.3389/fpls.2020.593047
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) (2014) Scientific opinion on dietary reference values for selenium. EFSA J 12:3846. https://doi.org/10.2903/j.efsa.2014.3846
EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst K-I, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Aggett P, Crous Bou M, Cubadda F, Ciccolallo L, de Sesmaisons Lecarre A, Fabiani L, Titz A, Naska A (2023) Scientific opinion on the tolerable upper intake level for selenium. EFSA J 21:7704. https://doi.org/10.2903/j.efsa.2023.7704
Egnér H, Riehm H, Domingo WR (1960) Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung Des Nährstoffzustandes Der Böden. II. K Lantbr Högsk. Ann 26:199–215
Egressy-Molnár O, Ouerdane L, Győrfi J, Dernovics M (2016) Analogy in selenium enrichment and selenium speciation between selenized yeast Saccharomyces cerevisiae and Hericium erinaceus (lion’s mane mushroom). LWT-Food Sci Technol 68:306–312. https://doi.org/10.1016/j.lwt.2015.12.028
EU 2020/2117 (2020) Concerning the renewal of the authorisation of selenomethionine produced by Saccharomyces cerevisiae CNCM I-3399 with the new name ‘selenised yeast Saccharomyces cerevisiae CNCM I-3399’ as a feed additive for all animal species, and repealing Regulation (EC) No 900/2009. http://data.europa.eu/eli/reg_impl/2020/2117/oj Accessed on 13 Sept 2023
Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R (2011) Selenium in human health and disease. Antioxid Redox Signal 14:1337–1383
Fairweather-Tait SJ, Collings R, Hurst R (2010) Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr 91:1484S-1491S. https://doi.org/10.3945/ajcn.2010.28674J
FAO (2022) UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT). Rome, Italy: FAO. https://www.fao.org/faostat/en/#home Accessed on 29 Jul 2022
FAO (2020) Standard operating procedure for soil organic carbon Walkley-Black method Titration and colorimetric method. FAO: Rome, Italy. https://www.fao.org/3/ca7471en/ca7471en.pdf. Accessed on 22 Mar 2023
Fox TE, Van den Heuvel EGHM, Atherton CA, Dainty JR, Lewis DJ, Langford NJ, Crews HM, Luten JB, Lorentzen M, Sieling FW, van Aken-Schneyder P, Hoek M, Kotterman MJJ, van Dael P, Fairweather-Tait SJ (2004) Bioavailability of selenium from fish, yeast and selenate: a comparative study in humans using stable isotopes. Eur J Clin Nutr 58:343–349. https://doi.org/10.1038/sj.ejcn.1601787
Galić L, Galić V, Ivezić V, Zebec V, Jović J, Đikić M, Filipović A, Manojlović M, Almås ÅR, Lončarić Z (2023) Modelling leverage of different soil properties on selenium water-solubility in soils of Southeast Europe. Agronomy 13:824. https://doi.org/10.3390/agronomy13030824
Galić L, Vinković T, Ravnjak B, Lončarić Z (2021) Agronomic biofortification of significant cereal crops with Selenium—A review. Agronomy 11:1015. https://doi.org/10.3390/agronomy11051015
Garousi F, Domokos-Szabolcsy É, Jánószky M, Balláné Kovács A, Veres S, Soós Á, Kovács B (2017) Selenoamino acid-enriched green pea as a value-added plant protein source for humans and livestock. Plant Foods Hum Nutr 72:168–175. https://doi.org/10.1007/s11130-017-0606-5
Gissel-Nielsen G, Gupta UC, Lamand M, Westermarck T (1984) Selenium in soils and plants and its importance in livestock and human nutrition. Adv Agron 37:397–460. https://doi.org/10.1016/S0065-2113(08)60459-9
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Hart DJ, Fairweather-Tait SJ, Broadley MR, Dickinson SJ, Foot I, Knott P, McGrath SP, Mowat H, Norman K, Scott PR, Stroud JL, Tucker M, White PJ, Zhao FJ, Hurst R (2011) Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem 126:1771–1778. https://doi.org/10.1016/j.foodchem.2010.12.079
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318. https://doi.org/10.1016/j.jtemb.2005.02.009
Hawkesford MJ, Zhao FJ (2007) Strategies for increasing the selenium content of wheat. J Cereal Sci 46:282–292. https://doi.org/10.1016/j.jcs.2007.02.006
Hinojosa Reyes L, Marchante Gayón J, García Alonso J, Sanz-Medel A (2003a) Determination of selenium in biological materials by isotope dilution analysis with an octapole reaction system ICP-MS. J Anal Spectrom 18:11–16. https://doi.org/10.1039/B209213A
Hinojosa Reyes L, Marchante Gayón J, García Alonso J, Sanz-Medel A (2003b) Quantitative speciation of selenium in human serum by affinity chromatography coupled to post-column isotope dilution analysis ICP-MS. J Anal at Spectrom 18:1210–1216. https://doi.org/10.1039/B305455A
Huerta VD, Sánchez MLF, Sanz-Medel A (2004) Quantitative selenium speciation in cod muscle by isotope dilution ICP-MS with a reaction cell: comparison of different reported extraction procedures. J Ana Spectrom 19:644–648. https://doi.org/10.1039/B313826G
Ip C, Dong Y, Ganther HE (2002) New concepts in selenium chemoprevention. Cancer Metastasis Rev 21:281–289. https://doi.org/10.1023/A:1021263027659
ISO 11261:1995 (1995) Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland. https://www.iso.org/standard/19239.html. Accessed on 22 Mar 2023
ISO 10693: (1997) Edition, May 1997 - Soil quality - Determination of carbonate content - Volumetric method (ISO 10693:1995). International Organization for Standardization: Geneva, Switzerland, 1997. https://www.iso.org/standard/18781.html Accessed on 22 Mar 2023
ISO 13536:1995- Soil quality - Determination of the potential cation exchange capacity and exchangeable cations using barium chloride solution buffered at pH = 8,1. International Organization for Standardization: Geneva, Switzerland, 1995. https://www.iso.org/standard/22180.html. Accessed on 22 Mar 2023
ISO 12914:2012 (2012) Soil quality — Microwave-assisted extraction of the aqua regia soluble fraction for the determination of elements. International Organization for Standardization: Geneva, Switzerland. https://www.iso.org/standard/52171.html. Accessed on 22 Mar 2023
Kápolna E, Gergely V, Dernovics M, Illés A, Fodor P (2007) Fate of selenium species in sesame seeds during simulated bakery process. J Food Eng 79:494–501. https://doi.org/10.1016/j.jfoodeng.2006.01.075
Kápolna E, Hillestrøm PR, Laursen KH, Husted S, Larsen EH (2009) Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem 115:1357–1363. https://doi.org/10.1016/j.foodchem.2009.01.054
Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R (1999) Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 65:4734–4740. https://doi.org/10.1128/AEM.65.11.4734-4740.19
Khanam A, Platel K (2016) Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem 194:1293–1299. https://doi.org/10.1016/j.foodchem.2015.09.005
Kim YB, Garbisu C, Pickering IJ, Prince RC, George GN, Cho M-J, Wong JH, Buchanan BB (2003) Thioredoxin h overexpressed in barley seeds enhances selenite resistance and uptake during germination and early seedling development. Planta 218:186–191. https://doi.org/10.1007/s00425-003-1102-8
Kirby JK, Lyons GH, Karkkainen MP (2008) Selenium speciation and bioavailability in biofortified products using species-unspecific isotope dilution and reverse phase ion pairing-inductively coupled plasma-mass spectrometry. J Agric Food Chem 56:1772–1779. https://doi.org/10.1021/jf073030v
Kojouri GA, Sadeghian S, Mohebbi A, Dezfouli MRM (2012) The effects of oral consumption of selenium nanoparticles on chemotactic and respiratory burst activities of neutrophils in comparison with sodium selenite in sheep. Biol Trace Elem Res 146:160–166. https://doi.org/10.1007/s12011-011-9241-4
Koszta G, Kacska Z, Szatmári K, Szerafin T, Fülesdi B (2012) Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac Surgery. J Anesth 26:812–821. https://doi.org/10.1007/s00540-012-1454-y
Kovács Z, Soós Á, Kovács B, Kaszás L, Elhawat N, Razem M, Veres S, Fári MG, Koroknai J, Alshaal T, Domokos-Szabolcsy É (2023) Nutrichemical alterations in different fractions of multiple-harvest alfalfa (Medicago sativa L.) green biomass fortified with various selenium forms. Plant Soil 487:173–195. https://doi.org/10.1007/s11104-023-05917-8
Kowalska I, Smoleń S, Czernicka M, Halka M, Kęska K, Pitala J (2020) Effect of selenium form and salicylic acid on the accumulation of selenium speciation forms in hydroponically grown lettuce. Agriculture 10:584. https://doi.org/10.3390/agriculture10120584
Lakanen E, Erviö R (1971) A comparison of eight extractants for the determination of plant available microelements in soils. Acta Agric Fenn 123:223–232
Lavu RVS, Du Laing G, Van De Wiele T, Pratti VL, Willekens K, Vandecasteele B, Tack F (2012) Fertilizing soil with selenium fertilizers: impact on concentration, speciation, and Bioaccessibility of Selenium in Leek (Allium ampeloprasum). J Agric Food Chem 60:10930–10935. https://doi.org/10.1021/jf302931z
Lindblom SD, Valdez-Barillas JR, Fakra SC, Marcus MA, Wangeline AL, Pilon-Smits EAH (2013) Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ Exp Bot 88:33–42. https://doi.org/10.1016/j.envexpbot.2011.12.011
Lončarić Z, Ivezić V, Kerovec D, Rebekić A (2021) Foliar Zinc-Selenium and nitrogen fertilization affects content of Zn, Fe, Se, P, and cd in Wheat Grain. Plants 10:1549. https://doi.org/10.3390/plants10081549
Long Z, Yuan L, Hou Y, Bañuelos GS, Liu Y, Pan L, Liu X, Yin X (2018) Spatial variations in soil selenium and residential dietary selenium intake in a selenium-rich county, Shitai, Anhui, China. J Trace Elem Med Biol 50:111–116. https://doi.org/10.1016/j.jtemb.2018.06.019
Luo Y, Chen G, Deng X, Cai H, Fu X, Xu F, Xiao X, Huo Y, Luo J (2022) Speciation of Selenium in Selenium-Enriched foods by high-performance liquid chromatography-inductively coupled plasma-Tandem Mass Spectrometry. Separations 9:242. https://doi.org/10.3390/separations9090242
Maneetong S, Chookhampaeng S, Chantiratikul A, Chinrasri O, Thosaikham W, Sittipout R, Chantiratikul P (2013) Hydroponic cultivation of selenium-enriched kale (Brassica oleracea var. alboglabra L.) seedling and speciation of selenium with HPLC–ICP-MS. Microchem J 108:87–91. https://doi.org/10.1016/j.microc.2013.01.003
Marschall TA, Bornhorst J, Kuehnelt D, Schwerdtle T (2016) Differing cytotoxicity and bioavailability of selenite, methylselenocysteine, selenomethionine, selenosugar 1 and trimethylselenonium ion and their underlying metabolic transformations in human cells. Mol Nutr Food Res 60:2622–2632. https://doi.org/10.1002/mnfr.201600422
Mazej D, Falnoga I, Veber M, Stibilj V (2006) Determination of selenium species in plant leaves by HPLC–UV–HG-AFS. Talanta 68:558–568. https://doi.org/10.1016/j.talanta.2005.04.056
McNeal JM, Balistrieri LS (1989) Geochemistry and occurrence of selenium: an overview. In: Jacobs LW (ed) Selenium in Agriculture and the Environment. Soil Science Society of America, Madison, WI, pp 1–13. https://doi.org/10.2136/sssaspecpub23.c1
Mechora Å, Germ M, Stibilj V (2012) Selenium compounds in selenium-enriched cabbage. Pure Appl Chem 84:259–268. https://doi.org/10.1351/PAC-CON-11-07-19
Montes-Baýon M, JośeDíaz Molet M, González EB, Sanz-Medel A (2006) Evaluation of different sample extraction strategies for selenium determination in seleniumenriched plants (Allium sativum and Brassica juncea) and Se speciation by HPLC– ICP-MS. Talanta 68:1287–1293. https://doi.org/10.1016/j.talanta.2005.07.040
Moreb N, Murphy A, Jaiswal S, Jaiswal AK (2020) Chap. 3 – Cabbage. In: Jaiswal AK (ed) Nutritional composition and antioxidant properties of fruits and vegetables. Academic Press, Cambridge, Massachusetts, USA, pp 33–54. https://doi.org/10.1016/B978-0-12-812780-3.00003-9
Moreno Rodriguez M, Cala Rivero V, Jiménez Ballesta R (2005) Selenium distribution in topsoils and plants of a semi-arid Mediterranean Environment. Environ Geochem Health 27:513–519. https://doi.org/10.1007/s10653-005-8625-9
Muleya M, Young SD, Reina SV, Ligowe IS, Broadley MR, Joy EJM, Chopera P, Bailey EH (2021) Selenium speciation and bioaccessibility in Se-fertilised crops of dietary importance in Malawi. J Food Comp Anal 98:103841. https://doi.org/10.1016/j.jfca.2021.103841
Nithiyanantham LS, Selvakumar S, Siddhuraju P (2012) Total phenolic content and antioxidant activity of two different solvent extracts from raw and processed legumes, Cicer arietinum L. and Pisum sativum L. J Food Comp Anal 27:52–60. https://doi.org/10.1016/j.jfca.2012.04.003
Ogra Y, Kitaguchi T, Ishiwata K, Suzuki N, Iwashita Y, Suzuki KT (2007) Identification of selenohomolanthionine in selenium-enriched Japanese pungent radish. J Anal Spectrom 22:1390–1396. https://doi.org/10.1039/B707348H
Oliveira VCD, Faquin V, Guimarães KC, Andrade FR, Pereira J, Guilherme LRG (2018) Agronomic biofortification of carrot with selenium. Ciência Agrotecnol 42:138–147. https://doi.org/10.1590/1413-70542018422031217
Oremland RS, Hollibaugh JT, Maest AS, Presser TS, Miller LG, Culberston CW (1989) Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl Environ Microbiol 55:2333–2343. https://doi.org/10.1128/aem.55.9.2333-2343.1989
Ouerdane L, Both EB, Xiang J, Yin H, Kang Y, Shao S, Kiszelák K, Jókai Z, Dernovics M (2020) Water soluble selenometabolome of Cardamine violifolia. Metallomics 12:2032–2048. https://doi.org/10.1039/d0mt00216j
Pezzarossa B, Rosellini I, Borghesi E, Tonutti P, Malorgio F (2014) Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci Hortic 165:106–110. https://doi.org/10.1016/j.scienta.2013.10.029
Phiri FP, Ander EL, Bailey EH, Chilima B, Chilimba AD, Gondwe J, Joy EJ, Kalimbira AA, Kumssa DB, Lark RM (2019) The risk of selenium deficiency in Malawi is large and varies over multiple spatial scales. Sci Rep 9:6566. https://doi.org/10.1038/s41598-019-43013-z
Pilon-Smits EAH (2019) On the Ecology of Selenium Accumulation in plants. Plants 8:197. https://doi.org/10.3390/plants8070197
Pilon-Smits EAH, Le Duc D (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20:207–212. https://doi.org/10.1016/j.copbio.2009.02.001
Pilon-Smits EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N (1999) Overexpression of ATP sulfurylase in Indian Mustard leads to increased selenate uptake, reduction, and Tolerance. Plant Physiol 119:123–132. https://doi.org/10.1104/pp.119.1.123
Poblaciones MJ, Rengel Z (2018) The effect of processing on Pisum sativum L. biofortified with sodium selenate. J Plant Nutr Soil Sci 181:932–937. https://doi.org/10.1002/jpln.201800251
Poblaciones MJ, Rodrigo S, Santamaria O, Chen Y, McGrath SP (2014a) Selenium accumulation and speciation in biofortified chickpea (Cicer arietinum L.) under Mediterranean conditions. J Sci Food Agric 94:1101–1106. https://doi.org/10.1002/jsfa.6372
Poblaciones MJ, Rodrigo S, Santamaria O, Chen Y, McGrath SP (2014b) Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: from grain to cooked pasta. Food Chem 146:378–384. https://doi.org/10.1016/j.foodchem.2013.09.070
Pyrzynska K (2009) Selenium speciation in enriched vegetables. Food Chem 114:1183–1191. https://doi.org/10.1016/j.foodchem.2008.11.026
R Core Team (2022) R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.R-project.org/ Accessed on 22 Oct 2022
Ragályi P, Takács T, Füzy A, Uzinger N, Dobosy P, Záray G, Szűcs-Vásárhelyi N, Rékási M (2021) Effect of Se-Enriched Irrigation Water on the Biomass Production and Elemental Composition of Green Bean, Cabbage, Potato and Tomato. Plants 10:2086. https://doi.org/10.3390/plants10102086
Ragályi P, Takács T, Füzy A, Uzinger N, Dobosy P, Záray G, Szűcs-Vásárhelyi N, Rékási M (2022) Effect of Se-Enriched Irrigation Water and Soil texture on Biomass Production and Elemental Composition of Green Pea and Carrot and their contribution to Human Se Intake. Agriculture 12:496. https://doi.org/10.3390/agriculture12040496
Rayman MP (2004) The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr 92:557–573. https://doi.org/10.1079/BJN20041251
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100:254–268. https://doi.org/10.1017/S0007114508939830
Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR (2004) A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol 18:69–74. https://doi.org/10.1016/j.jtemb.2004.03.004
Sánchez-Rodas D, Mellano F, Martínez F, Palencia P, Giráldez I, Morales E (2016) Speciation analysis of Se-enriched strawberries (Fragaria Ananassa Duch) cultivated on hydroponics by HPLC-TR-HG-AFS. Microchem J 127:120–124. https://doi.org/10.1016/j.microc.2016.02.017
Schiavon M, Dall’Acqua S, Mietto A, Pilon-Smits EA, Sambo P, Masi A, Malagoli M (2013) Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L). J Agric Food Chem 61:10542–10554. https://doi.org/10.1021/jf4031822
Schiavon M, Nardi S, Vecchia Fd, Ertani A (2020) Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 453:245–270. https://doi.org/10.1007/s11104-020-04635-9
Schiavon M, Pilon-Smits EA (2017) The fascinating facets of plant selenium accumulation - biochemistry, physiology, evolution and ecology. New Phytol 213:1582–1596. https://doi.org/10.1111/nph.14378
Schrauzer GN (2000) Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr 130:1653–1656. https://doi.org/10.1093/jn/130.7.1653
Shao S, Mi X, Ouerdane L, Lobinski R, García-Reyes JF, Molina-Díaz A, Vass A, Dernovics M (2014) Quantification of Se-Methylselenocysteine and its γ-Glutamyl derivative from naturally Se-Enriched Green Bean (Phaseolus vulgaris Vulgaris) after HPLC-ESI-TOF-MS and Orbitrap MSn-Based identification. Food Anal Methods 7:1147–1157. https://doi.org/10.1007/s12161-013-9728-z
Slekovec M, Goessler W (2005) Accumulation of selenium in natural plants and selenium supplemented vegetable and selenium speciation by HPLC-ICPMS. Chem Speciat Bioavailab 17:63–73. https://doi.org/10.3184/095422905782774919
Smoleń S, Sady W (2009) The effect of various nitrogen fertilization and foliar nutrition regimes on the concentrations of sugars, carotenoids and phenolic compounds in carrot (Daucus carota L). Sci Hort 120:315–324. https://doi.org/10.1016/j.scienta.2008.11.029
Smoleń S, Skoczylas Ł, Ledwożyw-Smoleń I, Rakoczy R, Kopeć A, Piątkowska E, Bieżanowska-Kopeć R, Koronowicz A, Kapusta-Duch J (2016) Biofortification of Carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front Plant Sci 7:730. https://doi.org/10.3389/fpls.2016.00730
Smrkolj P, Germ M, Krett I, Stibilj V (2006) Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J Exp Bot 57:3595–3600. https://doi.org/10.1093/jxb/erl109
Stadlober M, Sager M, Irgolic KJ (2001) Effects of selenate supplemented fertilisation on the selenium level of cereals — identification and quantification of selenium compounds by HPLC–ICP–MS. Food Chem 73:357–366. https://doi.org/10.1016/S0308-8146(01)00115-7
Steen A, Strøm T, Bernhoft A (2008) Organic selenium supplementation increased selenium concentrations in ewe and newborn lamb blood and in slaughter lamb meat compared to inorganic selenium supplementation. Acta Vet Scand 50:7. https://doi.org/10.1186/1751-0147-50-7
Stroud JL, Li HF, Lopez-Bellido FJ, Broadley MR, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, Mowat H, Norman K, Scott P, Tucker M, White PJ, McGrath SP, Zhao FJ (2010) Impact of sulphur fertilisation on crop response to selenium fertilisation. Plant Soil 332:31–40. https://doi.org/10.1007/s11104-009-0230-8
Tan Q, Zhang L, Grant J, Cooper P, Tegeder M (2010) Increased phloem transport of S-Methylmethionine positively affects Sulfur and Nitrogen metabolism and seed development in pea plants. Plant Physiol 154:1886–1896. https://doi.org/10.1104/pp.110.166389
Terry N, Zayed AM, De Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432. https://doi.org/10.1146/annurev.arplant.51.1.401
Thiry C, Ruttens A, De Temmerman L, Schneider YJ, Pussemier L (2012) Current knowledge in species-related bioavailability of selenium in food. Food Chem 130:767–784. https://doi.org/10.1016/j.foodchem.2011.07.102
Tomei FA, Barton LL, Lemanski CL, Zocco TG, Fink NH, Sillerud LO (1995) Transformation of selenate and selenite to elemental selenium byDesulfovibrio desulfuricans. J Ind Microbiol 14:329–336. https://doi.org/10.1007/BF01569947
Tóth RJ, Csapó J (2018) The role of selenium in nutrition—A review. Acta Univ Sapientiae Aliment 11:128–144. https://doi.org/10.2478/ausal-2018-0008
Valdez Barillas JR, Quinn CF, Freeman JL, Lindblom SD, Fakra SC, Marcus MA, Gilligan TM, Alford ÉR, Wangeline AL, Pilon-Smits EA (2012) Selenium distribution and speciation in the hyperaccumulator Astragalus bisulcatus and associated ecological partners. Plant Physiol 159:1834–1844. https://doi.org/10.1104/pp.112.199307
Van Hoewyk D, Garifullina GF, Ackley AR, Abdel-Ghany SE, Marcus MA, Fakra S, Ishiyama K, Inoue E, Pilon M, Takahashi H, Pilon-Smits EA (2005) Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiol 139:1518–1528. https://doi.org/10.1104/pp.105.068684
Van Hoewyk D, Takahashi H, Hess A, Tamaoki M, Pilon-Smits EAH (2008) Transcriptome and biochemical analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 132:236–253. https://doi.org/10.1111/j.1399-3054.2007.01002.x
Vogrinčič M, Cuderman P, Kreft I, Stibilj V (2009) Selenium and its species distribution in above-ground plant parts of Selenium Enriched Buckwheat (Fagopyrum esculentum Moench). Anal Sci 25:1357–1363. https://doi.org/10.2116/analsci.25.1357
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42:1524–1533. https://doi.org/10.1016/j.freeradbiomed.2007.02.013
Whanger PD (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21:223–232. https://doi.org/10.1080/07315724.2002.10719214
White PJ (2018) Selenium metabolism in plants. BBA-Gen Subj 1862:2333–2342. https://doi.org/10.1016/j.bbagen.2018.05.006
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis; Springer, New York, ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org Accessed on 22 June 2021
Wiesner-Reinhold M, Schreiner M, Baldermann S, Schwarz D, Hanschen FS, Kipp AP, Rowan DD, Bentley-Hewitt KL, McKenzie MJ (2017) Mechanisms of Selenium Enrichment and Measurement in Brassicaceous Vegetables, and their application to Human Health. Front Plant Sci 8:1365. https://doi.org/10.3389/fpls.2017.01365
Wu L, Emberg A, Biggar JA (1994) Effects of elevated selenium concentration on selenium accumulation and nitrogen fixation symbiotic activity of Melilotus indica L. Ecotoxicol Environ Saf 27:50–63. https://doi.org/10.1006/eesa.1994.1006
Yoshida M, Sugihara S, Inoue Y, Chihara Y, Kondo M, Miyamoto S, Sukcharoen B (2005) Composition of chemical species of selenium contained in selenium-enriched shiitake mushroom and vegetables determined by high performance liquid chromatography with inductively coupled plasma mass spectrometry. J Nutr Sci Vitaminol 51:194–199. https://doi.org/10.3177/jnsv.51.194
Yuan Z, Long W, Liang T, Zhu M, Zhu A, Luo X, Fu L, Hu Z, Zhu R, Wu X (2023) Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 486:87–101. https://doi.org/10.1007/s11104-022-05567-2
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292. https://doi.org/10.1007/s004250050402
Funding
Open access funding provided by ELKH Centre for Agricultural Research. This research was funded by the National Research Development and Innovation Office, Grant No.: NVKP_16-1-2016-0044 and Grant No.: 2021 − 1.2.4-TÉT-2021-00035, and by the Eötvös Loránd Research Network ELKH, Grant No.: SA-26/2021.
Author information
Authors and Affiliations
Contributions
P.R. contributed to the implementation of the experiment, prepared the manuscript and conducted the statistical data analyses and visualisation; T.T. contributed to the implementation of the experiment and the compilation of Materials and Methods; Á.S. contributed to the compilation of Materials and Methods and Results, and conducted speciation analyses and visualisation; B.K. conducted speciation analyses; M.D. contributed to the speciation analyses and proofread the manuscript; Z.L. contributed to the concept of the study; P.D. was the leader of the project and performed analytical work; G.Z. contributed to the concept of the study and proofread the manuscript; M.R. contributed to the design and implementation of the experiment and proofread the manuscript. All authors have read and approved the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Fangjie Zhao.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.00 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ragályi, P., Takács, T., Soós, Á. et al. Quantitative analysis of selenium species in the edible parts of cabbage, carrot, tomato and green pea treated with selenate-enriched irrigation water. Plant Soil 496, 341–360 (2024). https://doi.org/10.1007/s11104-023-06365-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06365-0