Abstract
Background and aims
Wheat growth and productivity need an exceptional approach to resist the deleterious effects of salt stress.
Methods
This study proposed to assess the effectiveness of the exogenous application of plant growth-promoting rhizobacteria (PGPR; i.e., Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446) at a rate of 950 g ha−1 and foliar application of zinc oxide nanoparticles (ZnO-NPs; 500 mg L−1) against irrigation with saline (from a groundwater well) and fresh water (from the Nile River water) of wheat (Triticum aestivum L.) in sodic-saline soil during 2021 and 2022 growing seasons under open field conditions.
Results
The integrated application of PGPR and ZnO-NPs protected wheat plants against irrigation with saline water through increasing antioxidant enzyme activities, i.e., catalase (47%), peroxidase (102%), and superoxide dismutase (106%), and K+ uptake (27%) over control. Conversely, higher stress mitigation through the integrated application was illustrated by a considerable decline in electrolyte leakage (−62%), proline (−39%), MDA (−56%), and H2O2 levels (−60%). The N uptake by wheat grains increased by 57% upon treating plants with PGPR+ZnO-NPs, which also increased the Zn contents in grain and straw by 117% and 72%, respectively. Also, PGPR+ZnO-NPs increased the activity of soil urease and dehydrogenase by 80% and 232%, respectively, in plots irrigated with saline water.
Conclusion
The results of the present investigation suggest the use of the integrated application of PGPR and ZnO-NPs to protect wheat plants against salinity of soil and/ or irrigation water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of the world’s population has increased from two billion people in 1920 to eight billion people in 2022, with about 10 billion people expected by 2050 (Sadigov 2022), which causes an urgent augment in food demand. So, wheat production must be boosted from roughly 1% to 2.5% by 2050 (FAO 2021). Wheat (Triticum aestivum) is considered the most staplecrop among cereals in feeding as long as food security globally (Grote et al. 2021). Flour extracted from wheat is used in the production of bread, pasta, and other bakery products (Cappelli and Cini 2021). The objective is to increment wheat productivity and decline costs, reaching the highest quality and quantity of production (Erenstein et al. 2022). Nevertheless, there is an enormous need to boost wheat yield to meet the ever-increasing demand for food by 2050, while land continues to lose 1 to 2% due to salinity annually (Wheeler and Von Braun 2013).
Soil salinity is a severe environmental stress that limits the growth and productivity of crop plants (Zou et al. 2016). Worldwide, about 25% of irrigated lands are strictly injured by salt accumulation and are likely to reach up to 50% by the year 2050, whereas more than 424 million hectares of topsoil (0–30 cm) and 833 million hectares of subsoil (30–100 cm) are salt-affected, of which 85% of salt-affected topsoil and 62% of salt-affected subsoil are saline (McGeorge 1954). Furthermore, the lack of available water resources for the irrigation of field crops has forced the farmers to irrigate the crops with low-quality water, mainly characterized by a high degree of salinity (Munns 2002). Increasing salt-affected soil results in global food insecurity and considerably decreases agricultural productivity due to the accumulation of soluble salts in the rhizosphere region, which eventually cause cell death and the collapse of the entire plant due to oxidative stress and membrane instability owing to lipid peroxidation (Flowers et al. 2010). The exponential increase in population annually resulted in the need to reclaim the salt-affected soils and to discover best management practices that control this issue to secure agricultural production to address this problem (Munns et al. 2006).
Several approaches for combating soil salinity have been proposed, and the effectiveness of these approaches has been investigated to address this problem. Lately, the exploitation of plant growth-promoting rhizobacteria (PGPR) as seed inoculants under highly saline conditions has acquired interest as an eco-friendly sustainable approach for attaining higher crop productivity grown in salt-affected soil by colonizing plant roots to control abiotic stresses (Ahmad et al. 2015). Many rhizobacteria species, i.e., Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Bacillus, and Serratia, have been explored to enhance plant growth under salt-affected soil. These PGPR can enhance plant tolerance to salinity stress through various mechanisms that involve both direct and indirect effects on the plant. For instance, PGPR can counter the adverse impacts of the ion toxicity due to the accumulation of Na+ and Cl− ions in plant tissues by promoting the exclusion of these toxic ions from the roots, reducing their uptake by the plant. Additionally, some PGPR can also enhance the uptake and accumulation of essential ions like K+, which helps maintain a more favorable ion balance in plant cells (Gerhardt et al. 2017). Also, PGPR can synthesize and release plant growth-promoting hormones such as auxins, cytokinins, and gibberellins. These hormones play vital roles in promoting root growth, nutrient uptake, and overall plant growth, helping plants cope with salinity stress (Tsukanova et al. 2017). PGPR can activate systemic tolerance mechanisms in plants, meaning that the enhanced tolerance not only affects the site of PGPR colonization but also other parts of the plant that are not directly in contact with the bacteria. This systemic response helps the entire plant to better withstand salinity stress (Figueiredo et al. 2016). Salinity stress leads to the accumulation of reactive oxygen species (ROS), which cause oxidative damage to plant cells. PGPR can induce the production of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), which help neutralize ROS and protect plant cells from oxidative stress (Nivetha et al. 2021). Some PGPR can produce compatible solutes or osmolytes like proline and glycine betaine, which act as osmoprotectants. These compounds help maintain cellular water balance, protect proteins, and stabilize membranes under saline conditions (Barnawal et al. 2019). Certain PGPR strains can enhance the water use efficiency of plants, meaning that they can achieve better growth and yield under limited water availability, including saline conditions (Pereira et al. 2020). By colonizing the root surface, PGPR can compete with and suppress the growth of pathogenic microbes. This biocontrol activity indirectly helps in reducing plant stress caused by salt-sensitive pathogens (Zahoor et al. 2022). It’s important to note that the specific mechanisms employed by PGPR may vary depending on the bacterial strain, the plant species, and the prevailing environmental conditions. Moreover, the effectiveness of PGPR in enhancing plant tolerance to salinity stress can also depend on factors such as the initial stress severity and the timing of PGPR application.
The foliar spraying of several plant crops with different essential/beneficial elements in their nanoscale to mitigate the salt-induced negative impacts has been significantly enhanced by nanotechnology (Singh et al. 2021). Nanoparticles are usually applied in low concentrations, which are cost-effective and eco-friendly (Liu et al. 2022). Zinc oxide nanoparticles (ZnO-NPs) have acquired great interest from agricultural scientists because of their possible usage as nano-fertilizers and nano-growth regulators to enhance the growth and productivity of crops, primarily owing to the efficient supply of zinc (Zn) and its uptake (Awan et al. 2021). Recently, several pieces of research confirmed that ZnO-NPs could be applied as an alleviator under salt stress conditions (Al Jabri et al. 2022; Zhang et al. 2021). It was proven its efficient role in antioxidants’ biosynthesis, enzymatic activity, and carbohydrates’ metabolism under saline stress conditions, resulting in improvements in biochemical and physiological properties, including sustaining water balance and accumulation of compatible solutes and protecting cells from ROS (reactive oxygen species) damage and ionic adjustment (Adil et al. 2022; Adrees et al. 2021; Faizan et al. 2021).
The current investigation aimed to assess the salt stress-alleviating capacity of combined PGPR and ZnO-NPs application by analyzing soil properties, the growth, physiology, and biochemistry of wheat plants, and its effect on yield-related traits, productivity, and nutrient uptake of wheat plants irrigated with saline and fresh water in sodic-saline soil.
Materials and methods
Experimental layout and growth conditions
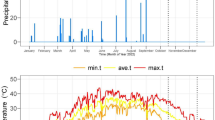
Open field experiments were conducted at the Elamaar township in the area of Sidi Salem (31° 07 N, 30° 57 E), Kafr El-sheik Governorate, Egypt, to assess the influence of the exogenous application of PGPR and ZnO-NPs on physiological and biochemical attributes alongside its effect on crop productivity of wheat plants (Triticum aestivum L., cv. Misr 1) irrigated with fresh and saline water in sodic-saline soil during two consecutive growing seasons. The experiments included four treatments, i.e., control (CK), PGPR, ZnO-NPs, and PGPR+ZnO-NPs, and two different types of irrigation water (i.e., fresh and saline water). The experimental layout was a split-block design with four replicates, where fresh water and saline water occupied the main plots, while PGPR and ZnO-NPs treatments represented the sub-main plots. The plot area was 10.5 m2 (3 × 3.5 m). The Wheat Research Department, Sakha, Kafr El-Sheikh, Egypt, provided us with wheat seeds. Seed sowing was applied at a rate of 140 kg ha−1 on 12 December 2020 and 04 December 2021. During soil preparation, experimental plots received calcium superphosphate at a rate of 107 kg ha−1 and 286 kg ha−1 of ammonium nitrate (33.5%). Calcium superphosphate was applied before cultivation, while ammonium nitrate was split into two equal portions, i.e., plants received the first half of ammonium nitrate before the 1st irrigation and the second half before the 2nd irrigation. Monthly data of maximum and minimum temperatures, rainfall level, and relative humidity were recorded by the nearest automated weather station from the experimental farm during the 2021 and 2022 growing seasons (Table S1).
Composite soil samples were collected by a stainless steel soil auger at a depth of 0–30 cm and analyzed physically and chemically before cultivation (Table S2). Soil type was clayey texture and classified as sodic-saline soil.
Fresh water was obtained from the Nile River, while the saline water was from a groundwater well. The Soil Improvement and Conservation Department, Agricultural Research Center, Giza, Egypt, characterized the irrigation waters (Table S3). Applied irrigation system was surface irrigation with a total of five irrigations with 3–4 weeks intervals as recommended by the Ministry of Agriculture and Land Reclamation, Egypt, with an average of 5842 m3 ha−1 per each irrigation.
Plant growth promoting rhizobacteria characterization
Four bacterial strains, i.e., Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446, were selected for their potent plant growth promoting characteristics and were provided by the Bacteriology Laboratory, Sakha Agricultural Research Station, Kafr El-Sheikh, Egypt. The standard growth cultures were a semi-solid malate medium for the A. lipoferum strain (Döbereiner and Day 1976) and a nutrient broth medium for the Bacillus strains (Atlas and Atlas 2004). The inoculums were set as peat-based inoculum, with 30 mL of 109 CFU mL−1 from each bacterial culture per 60 g of a sterilized carrier, and mixed thoroughly with wheat seeds before seed sowing at a rate of 950 g ha−1. Wheat seeds were surficial sterilized before the PGPR inoculation.
Source and properties of ZnO-NPs suspensions
The ZnO-NPs were obtained from the Faculty of Agriculture, El-Sadat Branch, Al-Azhar University, Egypt, with a purity of 99% (by XRF), spherical crystal structure, mean diameter size of 30–70 nm, and surface area of 40 m2 g−1. The ZnO-NPs suspension was prepared at the rate of 500 mg L−1 using a shaker (shaking power: 100 W and 40 kHz) for 30 min and applied to wheat plants via foliar application twice at 40 and 60 days after seed sowing using Tween-20 as sticking agent. The total applied volume of ZnO-NPs suspension was 500 mL per 1 m2, and control plants received the same amount of distilled water.
Measurements
Accumulation of ions in wheat leaves (mg g−1 DW)
A 3 g from the top-most fully expanded leaves at 80 days after sowing were randomly sampled and oven-dried at 75 °C for 72 h, and then crushed into a grind and were reduced to ash for Na+ and K+ extraction with 8 mL of digestion mixture HNO3:HClO4 (3:1 v/v). The ion concentrations in the leaves were assessed by an ion chromatograph with a conductivity detector (Shimazu, Japan), according to the method provided by (Yoshida et al. 2016).
Electrolyte leakage (EL, %)
The electrolyte leakage (EL) was assessed as explained by (Bajji et al. 2002). Firstly, at 80 days after seed sowing, 10 disks from the uppermost fully-expanded leaves were collected, washed with distilled water, and placed into test tubes containing distilled water for measuring the initial electrical conductivity (EC1) after incubation at 55 °C for 25 min. Secondly, the test tubes were heated to 100 °C for 10 min, and the electrical conductivity (EC2) was measured again. The EL was computed by the following equation:
Leaf relative water content (RWC, %)
The RWC in leaves of wheat plants was determined at 80 days after seed sowing using ten fully expanded leaves from the plant tip according to the method of (Barrs and Weatherley 1962). The collected samples were weighed to record the fresh weight (FW), placed into test tubes (10 mL) with 2 mL distilled water, and stored in the refrigerator at 4 °C for 24 h. The same samples were immersed in water till a constant weight to record the turgid weight (TW). The turgid leaves were dried in a ventilated oven at 75 °C for 24 h and weighed to record dried weight (DW). The RWC was calculated according to the following formula:
Hydrogen peroxide (H2O2, μmol g−1 FW)
A 1 g of top-most fully expanded leaves at 80 days after seed sowing were sampled according to the method of (Velikova et al. 2000) to determine H2O2 content colorimetrically after extraction by liquid N2 and trichloroacetic acid (TCA: 0.1%) and centrifugation at 12,000 g for 15 min. The concentration of the yellow color of the supernatant was measured at 390 nm by the model UV-160A spectrophotometer (Shimadzu, Japan).
Malondialdehyde (MDA; nmol g−1 FW)
Malondialdehyde is an end product of lipid peroxidation. The thiobarbituric acid test (TBA) was used to assess MDA content using the method of (Du and Bramlage 1992). At 80 days after seed sowing, 500 mg from the top-most fully expanded leaves were homogenized and ground in liquid N2 and hydro-acetone buffer (4:1 v/v). Later, 20% trichloroacetic acid (TCA) solution and 0.01% butyl hydroxyl toluene (BHT) were applied, and samples were incubated at 95 °C. Next to incubation, the homogenized samples were exposed to centrifugation at 10,000 g for 10 min. The absorbance was measured spectrophotometrically at 532 and 600 nm using the model UV-160A spectrophotometer (Shimadzu, Japan).
Proline content (mg 100 g−1 FW)
Proline is an essential osmolyte and osmoprotective compound. Leaf proline content was measured using the method developed by (Bates et al. 1973). Briefly, 0.5 g of the top-most fully expanded leaves at 80 days after seed sowing was ground with sulfuric acid (3%) and spun at 12,000 g for 5 min the solution was quantified by ninhydrin reagent. The obtained supernatant was then homogenized with toluene, and the absorbance was measured at 520 nm using the model UV-160A spectrophotometer (Shimadzu, Japan).
Enzymatic antioxidant assays
Catalase (CAT; EC: 1.11.1.6) is mostly catalyzing the H2O2 into H2O and O2. The measurement of CAT activity (μmol H2O2 min−1 g−1 FW) as obtained by (Aebi 1984). The analyze mixture contains a plant extract, 100 mM K-phosphate buffer (pH 7.0) and 75 mM H2O2. Therefore the absorbance was computed at 240 nm using UV–visible spectrophotometer. The reaction started by applying H2O2 and the absorbance was examined at 240 nm for 60 s. The activity of CAT enzyme was expressed as unit mg−1 protein. Superoxide dismutases (SOD; EC: 1.15.1.1) are metalloproteins. The activity of SOD (μmol H2O2 min−1 g−1 FW) was appraised by the 50% NBT reduction assay at 560 nm as stated by (Beauchamp and Fridovich 1971). In addition, the peroxidase (POD; EC: 1.11.1.7) activity (μmol H2O2 min−1 g−1 FW) was appraised by o-phenylenediamine as a chromogenic marker in the existence of H2O2 and enzyme extract at 417 nm as stated by (Vetter et al. 1958).
Chlorophyll pigments (mg g−1 FW)
Chlorophyll pigments were determined at 80 days after seed sowing using the top-most fully expanded leaves according to the method reported by (Lichtenthaler 1987). Briefly, 1 g of fresh leaf tissue was used to extract the photosynthetic pigments with 6 mL acetone 80%. Samples were incubated in the dark at room temperature overnight and then centrifuged at 12,000 rpm for 15 min. The absorbance of the supernatant was measured at 645, 663, and 470 nm using the model UV-160A spectrophotometer (Shimadzu, Japan). The contents of chlorophyll and carotenoid in the solution were computed as follows:
Stomatal conductance (Sc; mmol H2O m−2 s−1)
Ten top-most fully expanded leaves at 80 days after seed sowing were sampled randomly between 10:00 and 11:00 am to measure the stomatal conductance using AP4 porometer (Delta-T Devices Ltd., 130 Low Road, Burwell, Cambridge, CB25 0EJ, United Kingdom) on the abaxial (rb) and adaxial (ra) side using the following formula:
Crop yield
At maturity, ten wheat samples from each plot were randomly used for measuring number of grains per spike and 1000-grain weight (g). Also, 6 m2 from each plot was harvested to weight grain and straw yield. Biological yield was measured by straw and grain yields with regard to grain moisture (14%). Harvest index (%) was measured by the next formula:
Nutrient uptake
At harvest, 10 g of grains from each treatment were selected, air-dried, crushed, and set for laboratory analysis for grain N, P, and K content. The N, P, and K contents in the wheat grain were assessed by micro Kjeldahl’s by the method of (AOAC 2016), by a spectrophotometer, and flame photometer by the method of (Sparks et al. 1996), respectively. The content of Zn (mg kg−1) in grains and straw of wheat plants was determined in the finely ground samples after the digestion with the mixture of HNO3:HClO4 (3:1 v/v) using Atomic Absorption Spectrophotometer (AAS, PERKIN ELMER 3300) with a detection limit of 100 ppb (Sparks et al. 1996).
Soil urease and dehydrogenase enzymes activity
Urease activity (mg NH4+ g−1 dry soil d−1) was determined at 80 days after seed sowing using urea as a substrate by the spectrophotometric technique at 670 nm, as described by (Svane et al. 2020). Briefly, 10 g of humid soil was incubated with 2 mL methylbenzene, 11 mL 11% urea, and 25 mL citrate buffer (pH 6.7) for a day at 37 °C. A 2 mL purified soil solution, 2 mL C6H5NaO, and 2 mL NaClO were mixed and brought to 60 mL with sterilized distilled water. Absorbance was assessed at 565 nm by the model UV-160A spectrophotometer (Shimadzu, Japan). The dehydrogenase activity was measured by the technique of (Phale et al. 2019). Dehydrogenase activity (mg TPF g−1 dry soil d−1) was determined by using triphenyl tetrazolium chloride (TTC) as a substrate, while the triphenyl tetrazolium chloride solution (0.5–0.6 g/100 mL) was miscellaneous with 6 g of humid soil and incubated for 24 h at 30 °C. Later, 50 mL of acetone was applied, and absorbance was assessed at 546 nm by the model UV-160A spectrophotometer (Shimadzu, Japan).
Statistical analysis
Data analysis was conducted using Microsoft Excel 2016 and the SPSS 25.0 software package (SPSS Inc., Chicago, IL, USA). The analysis of variance using one-way ANOVA was performed separately between treatments, seasons, or type of irrigation water. Separation of means was performed by post-hoc test (Tukey’s test), and significant differences were accepted at the level p ≤ 0.05. The data were presented as mean ± standard deviation.
Results
Chemical and biochemical traits of wheat irrigated with saline water in saline soil
Ions content in wheat leaves
Control (CK) wheat plants grown in sodic-saline soil showed their ability to absorb and accumulate Na+ in their tissues (8.85 mg g−1); however, higher Na+ content (11.72 mg g−1) was reported when saline water was utilized in the irrigation of plants (Fig. 1). The individual application of PGPR or ZnO-NPs illustrated their capacity to reduce the uptake and accumulation of Na+ in wheat tissues. Nevertheless, the best effect corresponded to the dual application of PGPR and ZnO-NPs, where Na+ content dropped from 8.85 mg g−1 (CK) to 8.15 mg g−1 when plants received fresh water and from 11.72 mg g−1 (CK) to 8.73 mg g−1 when saline water was applied. Similar results were noticed in both growing seasons. Contrariwise, soil- or water-derived salinity stress reduced the uptake of K+ by wheat roots. Irrigated CK plants with fresh water in sodic-saline soil exhibited a low K+ content (14.43 mg g−1), while irrigated plants with saline water revealed the lowest K+ content (11.69 mg g−1). Nonetheless, a considerable increase in K+ in wheat leaves was measured upon treating plants with the combination of PGPR and ZnO-NPs, recording 14.81 mg g−1 (for saline water) and 17.30 mg g−1 (for fresh water). Based on Na+ and K+ results, the K+/Na+ ratio was calculated. Results showed that CK plants irrigated with saline water revealed the lowest ratio (1.0), whereas irrigated CK plants with fresh water had a K+/Na+ ratio of 1.6. However, the single and combined applications of PGPR and ZnO-NPs displayed higher K+/Na+ ratios. The highest K+/Na+ ratio corresponded to irrigated plants with fresh water and received PGPR+ZnO-NPs (2.1), yet this ratio dropped to 1.7 upon irrigating plants with saline water.
Absorption and deposition of: A) Na+ and B) K+; C) K+/Na+ and D) electrolyte leakage (EL) in wheat leaves (Triticum aestivum L., cv. Misr 1) irrigated with saline and fresh water in sodic-saline soil after treating them with plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
The negative impact of increased uptake of Na+ due to soil and water salinity was clarified by the electrolyte leakage (EL) values. The CK plants irrigated with saline water in sodic-saline soil possessed an EL of 15.37%, while irrigated CK plants with fresh water resulted in an EL of 7.13%. However, treated plants with PGPR, ZnO-NPs, or their combination showed a marked reduction in the EL. For instance, irrigated plants with fresh water upon receiving PGPR+ZnO-NPs possessed an EL of 2.75%, while irrigated plants of the same treatment with saline water showed an EL of 5.86%.
Relative water content and contents of proline, H2O2, and malondialdehyde
The lowest relative water content (RWC) maintained by wheat leaves (70.3%) corresponded to irrigated CK plants with saline water in sodic-saline soil (Fig. 2). However, watering the CK plants with fresh water in sodic-saline soil revealed higher RWC (84.2%). Applied treatments, i.e., PGPR and ZnO-NPs, considerably improved the RWC in wheat leaves, recording higher RWCs. The combined application of PGPR+ZnO-NPs, especially, displayed the highest RWC of plants irrigated with saline water (80.0%) and fresh water (84.2%) in sodic-saline soil. Irrigated CK plants with fresh and saline water in sodic-saline soil exhibited the highest proline contents of 10.5 and 11.1 mg 100 g−1 FW, respectively (Fig. 2). Results revealed that the exogenous application of PGPR and ZnO-NPs, singularly or in combination, induced wheat plant tolerance to salinity by lowering the leaf proline content. For example, plants that received the combined application of PGPR and ZnO-NPs showed the lowest proline contents of 6.8 and 6.2 mg 100 g−1 FW when irrigated with saline and fresh water, respectively.
A) relative water content; B) proline content; C) H2O2 content; and D) malondialdehyde (MDA) content in wheat leaves (Triticum aestivum L., cv. Misr 1) irrigated with saline and fresh water in sodic-saline soil after treating them with plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
Similarly, the content of the oxidant H2O2 in leaves of wheat plants grown in sodic-saline soil substantially declined with treating plants with PGPR and ZnO-NPs, regardless of the type of irrigation water or application method (Fig. 2). The H2O2 content decreased from 4.23 μmol g−1 FW (for CK) to 1.68 μmol g−1 FW (for PGPR+ZnO-NPs) upon irrigating plants with saline water. However, a higher reduction in H2O2 content corresponded to the use of fresh water. For instance, H2O2 content changed from 2.58 μmol g−1 FW (for CK) to 1.39 μmol g−1 FW (for PGPR+ZnO-NPs) upon irrigating plants with fresh water.
Likewise, the level of lipid peroxidation of the bilayer in the cell membrane considerably increased upon irrigating plants with fresh water in sodic-saline soil; however, the highest reduction in lipid peroxidation corresponded to the applied treatments, mainly the combined application of PGPR+ZnO-NPs. The results of malondialdehyde (MDA) as an indicator of lipid peroxidation degree showed that the usage of saline water in irrigating plants had very negative impacts on plant growth, recording the highest MDA content (14.54 nmol g−1 FW). Nevertheless, a considerable reduction in the MDA content was reported upon treating plants with PGPR+ZnO-NPs, recording an MDA content of 6.44 nmol g−1 FW. In the case of fresh water, the MDA content dropped from 7.58 nmol g−1 FW (for CK) to 1.89 nmol g−1 FW (for the treatment of PGPR+ZnO-NPs).
Activity of antioxidant enzymes in wheat leaves
Cultivation of wheat plants in sodic-saline soil significantly lowered the activity of antioxidant enzymes (μmol H2O2 min−1 g−1 FW), i.e., SOD, CAT, and POD, especially when plants were irrigated with saline water (Fig. 3). The exogenous application of PGPR and ZnO-NPs significantly enhanced the activity of antioxidant enzymes, regardless of the type of irrigation water. The combined application of PGPR+ZnO-NPs showed the highest activity of the antioxidant enzymes. In the case of SOD, the CK plants irrigated with saline water had a SOD activity of 38.6, which increased to 79.5 upon treating plants with PGPR+ZnO-NPs. Likewise, the irrigated CK plants with fresh water showed a SOD activity of 60.5, while treated plants with PGPR+ZnO-NPs displayed the highest SOD activity of 106.9.
Activity of some antioxidant enzymes: A) superoxide dismutase (SOD); B) catalase (CAT); and C) peroxidase (POD) in leaves of wheat plants (Triticum aestivum L., cv. Misr 1) irrigated with saline and fresh water in sodic-saline soil after treating them with plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
Irrigated plants with fresh water in sodic-saline soil revealed higher CAT activity than those watered with saline water. Moreover, the application of PGPR and ZnO-NPs, individually or in combination, exhibited higher CAT activities than CK. While irrigating CK plants with saline water resulted in a CAT activity of 0.72, fresh water possessed a CAT activity of 0.99. The highest CAT activities (1.06 and 1.34) corresponded to the combined application of PGPR and ZnO-NPs upon irrigating plants with saline and fresh water, respectively.
The response of the POD enzyme to water−/ soil-derived salinity was similar to those of SOD and CAT. The POD activity was lowered upon growing plants in sodic-saline soil without any treatment, recording the lowest POD activity in plants irrigated with saline (0.50) or fresh water (0.96). The application of ZnO-NPs significantly increased the activity of POD, regardless of the type of irrigation water. Moreover, the combined application of PGPR and ZnO-NPs revealed the highest POD activity of 1.01 (for saline water) and 1.43 (for fresh water).
Alteration in photosynthetic machinery of wheat plants
The contents of photosynthetic pigments (mg g−1 FW) significantly lowered in the leaves of wheat plants grown in sodic-saline soil (Fig. 4). Nevertheless, using saline water in irrigating plants significantly reduced the contents of photosynthetic pigments compared to fresh water. However, this negative impact was alleviated by the exogenous addition of PGPR and ZnO-NPs. The chlorophyll a (chl a) content of the CK plants irrigated with saline water was 0.89 and increased to 1.11 upon treating plants with PGPR+ZnO-NPs. On the other hand, the CK plants of fresh water irrigation had chl a content of 1.30, while treating plants with PGPR+ZnO-NPs resulted in chl a concentration of 1.74.
Changes in: A) chlorophyll a (chl a); B) chlorophyll b (chl b); C) carotenoids contents; and D) stomatal conductance in wheat leaves (Triticum aestivum L., cv. Misr 1) irrigated with saline and fresh water in sodic-saline soil after treating them with plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
In the same line, irrigated plants with saline water displayed a chlorophyll b (chl b) content of 0.20 for untreated plants and 0.42 for plants that received PGPR+ZnO-NPs. Overall, the exogenous application of PGPR and ZnO-NPs, singularly or in combination, significantly increased the content of chl b, regardless of the type of irrigation water. Irrigated CK and treated plants with fresh water showed higher chl b contents than in the case of saline water. For instance, the CK plants had a chl b content of 0.61, while treated plants with PGPR+ZnO-NPs possessed a chl b content of 1.05.
All irrigated plants with fresh water exhibited higher carotenoid contents than those that received saline water, except for the treated plants with PGPR+ZnO-NPs. Also, all the treated plants with PGPR and ZnO-NPs reported higher carotenoid contents than the CK plants. The CK plants of saline water irrigation had carotenoid content of 0.32, while the combined application of PGPR+ZnO-NPs resulted in the carotenoid concentration of 0.84, the highest recorded carotenoid content. The CK plants irrigated with fresh water displayed carotenoid content of 0.46; however, this value increased to 0.71 upon treating plants with PGPR+ZnO-NPs.
The stomatal conductance (mmol H2O m−2 s−1) of the leaves of wheat plants showed similar responses. Irrigating wheat plants without any treatment (CK) showed a stomatal conductance of 38.79, while treated plants with PGPR and ZnO-NPs reported higher values. The highest stomatal conductance (44.32) corresponded to plants receiving PGPR+ZnO-NPs. Otherwise, treated and untreated plants irrigated with fresh water showed higher stomatal conductance than those watered with saline water. The CK plants of fresh water treatment revealed a stomatal conductance of 41.82, while a higher value (49.39) was reported for those grown in the presence of PGPR+ZnO-NPs.
Productivity of wheat plants under salinity stress
Yield characteristics
The productivity of wheat plants was significantly lowered in sodic-saline soil; however, using fresh water for irrigating plants largely mitigated this negative impact compared to the saline water (Table 1). The number of grains per spike increased significantly by treating plants with PGPR and ZnO-NPs, regardless of the type of irrigation water. The CK plants irrigated with saline water displayed a number of grains per spike of 39.0, while plants that received PGPR+ZnO-NPs had 44.2 grains per spike. These numbers increased by watering plants with fresh water; for example, the CK and PGPR+ZnO-NPs treatments exhibited 41.5 and 46.5 grains per spike, respectively. Likewise, the exogenous application of PGPR and ZnO-NPs significantly improved the weight of 1000-grain compared to the CK plants. Moreover, irrigated plants with fresh water showed higher weights of 1000-grain than those irrigated with saline water. The CK plants irrigated with saline and fresh water revealed 42.9 and 45.1 g of 1000-grain, respectively. On the other hand, treated plants with PGPR+ZnO-NPs in the presence of saline and fresh water irrigation reported 48.1 and 53.3 g of 1000-grain, respectively.
Irrigated wheat plants with fresh water in sodic-saline soil reported higher straw yields (ton ha−1) than those watered with saline water. Moreover, the application of PGPR and ZnO-NPs, individually or in combination, resulted in higher straw yields than the CK plants. Irrigated CK plants with saline water had a straw yield of 6.5, while those that received PGPR+ZnO-NPs showed a straw yield of 7.6. The straw yield of the CK and treated plants with PGPR+ZnO-NPs were 7.0 and 8.4, respectively, when fresh water was used. Similarly, the grain yield (ton ha−1) of the CK plants irrigated with saline water was 2.9, while irrigated ones with fresh water displayed a grain yield of 3.4. The exogenous application of PGPR and ZnO-NPs significantly increased the grain yield of plants watered with either saline or fresh water. However, the combined addition of PGPR and ZnO-NPs revealed the highest grain yield, i.e., 4.2 (for saline water) and 4.9 (for fresh water). Treated plants with PGPR and ZnO-NPs exhibited a higher harvest index (HI) than the CK plants. Also, irrigated plants with fresh water had higher HI than those watered with saline water. The HI of the CK plants irrigated with saline and fresh water was 31.0% and 32.6%, respectively. Treated plants with PGPR+ZnO-NPs displayed the highest HI of 35.7% (for saline water) and 36.9% (for fresh water).
NPK uptake
The contents of NPK (mg g−1) in seeds of wheat plants grown in sodic-saline soil showed high dependence on the type of irrigation water and applied treatments, i.e., PGPR and ZnO-NPs (Table 2). Results revealed that the content of K is the highest in wheat seeds, followed by N and P. The N content in seeds of plants irrigated with saline water in the presence of PGPR+ZnO-NPs was 1.57, while in the case of fresh water, it was 1.70. The CK plants revealed the lowest seed N content of both irrigation water types, i.e., 1.43 (for saline water) and 1.55 (for fresh water). Likewise, treating plants with the combined application of PGPR and ZnO-NPs resulted in the highest content of P of wheat seeds of plants irrigated with saline (0.82) and fresh water (0.98). The CK plants displayed seed P content of 0.62 (for saline water) and 0.82 (for fresh water). On the contrary, no significant differences in K content in wheat seeds were reported among treatments, except for the CK plants irrigated with saline water. The K content in seeds ranged between 2.45 and 2.70, except for the CK plants watered with saline water, which showed a K content of 1.87.
The exogenous application of PGPR and ZnO-NPs significantly increased the uptake and accumulation of NPK in seeds of wheat plants, either those irrigated with saline or fresh water in sodic-saline soil. K displayed the highest uptake rate, followed by N and P. The combined application of PGPR and ZnO-NPs revealed the highest uptake rate of NPK. Moreover, irrigated plants with fresh water showed higher uptake rates than those that were watered with saline water.
Zn content in wheat plants
The content of Zn (mg kg−1) in wheat grain and straw significantly increased upon treating plants with PGPR and ZnO-NPs (Fig. 5). Although higher Zn contents in wheat grain and straw were noticed for those plants irrigated with fresh water than saline water, the percent increase upon the PGPR and ZnO-NPs application was greater than irrigation with saline water than fresh water. In the case of saline water irrigation, the Zn content in grains increased from 11.53 (for CK) to 25.00 (for PGPR+ZnO-NPs), while irrigated plants with fresh water displayed grain Zn content of 21.37 (for CK) and 35.12 (for PGPR+ZnO-NPs). Higher Zn contents were measured in wheat straw than in wheat grains. The CK plants irrigated with saline water possessed a straw Zn content of 41.57, whereas treated plants with PGPR+ZnO-NPs had a straw Zn content of 71.50. Irrigated plants with fresh water revealed higher straw Zn contents; for instance, the CK plants exhibited a straw Zn content of 66.78, which increased to 88.66 upon treating plants with PGPR+ZnO-NPs.
Zinc content (mg kg−1) in: A) grains and B) straw of wheat plants (Triticum aestivum L., cv. Misr 1) irrigated with saline and fresh water in sodic-saline soil after treating them with plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
Soil enzyme activities
The CK plots displayed low soil urease (mg NH4+ g−1 dry soil d−1) and dehydrogenase (mg TPF g−1 dry soil d−1) activity, particularly in the case of saline irrigation water (Fig. 6). The CK plots irrigated with fresh water exhibited a urease activity of 116, which dropped to 85 upon irrigating wheat plants with saline water. However, urease activity significantly increased upon treating plants with PGPR and ZnO-NPs, regardless of the type of irrigation water. Nevertheless, irrigated plots with fresh water revealed higher urease activity than ones that received saline water. Considerably, the ZnO-NPs application improved the activity of urease compared to the CK and other treatments in both cases of irrigation wheat plants with either saline or fresh water. However, the highest significant increase in the urease activity corresponded to the combined application of PGPR and ZnO-NPs, recording urease activity of 153 (in the case of saline water irrigation) and 215 (in the case of fresh water irrigation). Likewise, dehydrogenase activity showed similar responses to soil salinity and irrigation with saline water. The CK plants irrigated with saline or fresh displayed dehydrogenase activity of 34 and 56, respectively. Nonetheless, treating plants with PGPR+ZnO-NPs revealed the highest dehydrogenase activity under irrigation with saline (113) and fresh (146) water. The improvement in the soil enzyme activities was greater when wheat plants were irrigated with fresh water than saline water.
Activity of soil enzymes: A) urease and B) dehydrogenase after cultivation of wheat plants (Triticum aestivum L., cv. Misr 1) in sodic-saline soil irrigated with saline and fresh water in the presence of plant growth promoting rhizobacteria (PGPR; Azospirillum lipoferum SP2, Bacillus coagulans NCAIM B.01123, Bacillus circulance NCAIM B.02324, and Bacillus subtilis MF497446), zinc oxide nanoparticles (ZnO-NPs) and their combination during two consecutive seasons (2021 and 2022). Different letters on the same columns of the same season are significant according to the Tukey’s test (P ≤ 0.05). Data are Means ± SD and n = 3
Discussion
Improvements in soil properties
PGPR induces ACC-deaminase activity that reduces ethylene stress on roots. It helps in releasing 1-aminocyclopropane-1-carboxylic acid (ACC)-deaminase, producing exopolysaccharides (EPS), and enhancing the activity of soil enzymes (e.g., urease and dehydrogenase) (Ghorai et al. 2021). Application of ZnO-NPs, as foliar spraying, slightly improved soil properties under the harsh conditions, leading to an improvement in the biosynthesis of auxin by plants, which stimulates cell division and elongation (El-Zohri et al. 2021). The utilization of ZnO-NPs is anticipated to bolster soil fertility, increase crop productivity, and enhance food quality. When ZnO-NPs are introduced into the soil, they can influence the soil components and subsequently impact plant structure. Even at a small fraction, ZnO-NPs significantly affect the soil’s physical and engineering properties owing to their substantial specific surface area and nanoporosity (Sheteiwy et al. 2021). These nanoparticles have demonstrated the capability to enhance soil moisture content, water retention, and overall structure. Furthermore, the incorporation of ZnO-NPs led to improved soil porosity and reduced bulk density, which creates more favorable conditions for root growth (Sheteiwy et al. 2021). Coupled application of PGPR and ZnO-NPs could increase the activity of soil enzymes, e.g., urease and dehydrogenase, which improved soil properties facilitating water and nutrients uptake and translocation into plant tissues, leading to positive reflects on controlling Na+ entry into plant cells under salt stress (Azmat et al. 2022). The application of PGPR significantly increased K+ influx into the leaf tissues, which counters the Na+ ion increasing the turgor pressure in the leaves due to cell membrane stability and decreased EL (Kumawat et al. 2023). Consequently, increased photosynthetic pigments, RWC, and stomata conductance improved due to the PGPR application, which increases plant growth under salt stress conditions (Neshat et al. 2022). However, the application of ZnO-NPs could stimulate the biosynthesis of indigenous plant growth regulators like indole-3-acetic acid (IAA) and gibberellic acid (GA3), which have controlling role in the improvement of biosynthesis of photosynthetic pigments and physiological and biochemical properties owing to sustain cell membrane stability under salt stress leading to quicken plant growth and development (Yasmin et al. 2021).
Osmotic stress alleviation and ROS scavenging
Combined application of PGPR and ZnO-NPs considerably improved osmoregulation levels in leaf tissues and stomatal conductance prompted by higher K+ absorption. Consequently, it positively influenced transpiration and increased photosynthetic capacity, conserving the K+/Na+ ratio and alleviating the negative effect of salt stress (Yasmin et al. 2021). Accordingly, retaining water balance, maintaining the cell membrane’s stability, scavenging ROS due to increased activity of antioxidant enzymes, and accumulation of compatible solutes that indirectly lead to a reduction in the degree of lipid peroxidation occurred (El-Saadony et al. 2022; Mokrani et al. 2020). The relationship between the accumulation of compatible solutes and lipid peroxidation in plants is closely linked to the role of compatible solutes in mitigating oxidative stress caused by various environmental factors, including salinity, drought, extreme temperatures, and heavy metal toxicity (Paliwal et al. 2021). Under stress conditions, increased ROS production can lead to oxidative stress. One of the most vulnerable targets of ROS is the cell membrane’s lipids, particularly polyunsaturated fatty acids. ROS attack these fatty acids, initiating lipid peroxidation, which generates lipid peroxides and other reactive lipid species. Compatible solutes serve as antioxidants and scavengers of ROS. They help neutralize and stabilize ROS, preventing them from causing damage to cellular components, including lipids. Compatible solutes act as free-radical scavengers, protecting lipids from peroxidation and reducing the oxidative damage to cell membranes. Also, the accumulation of compatible solutes helps maintain cellular osmotic balance, which reduces the extent of cellular dehydration during stress. This, in turn, reduces the production of ROS, limiting the potential for lipid peroxidation (Helaly et al. 2017). The PGPR application in hyper-saline environmental conditions can modify plant physiology by persuading systemic tolerance to stimulate growth under salt stress. The PGPR improved the root exudates in the rhizosphere, led to the production of phytohormones, e.g., auxins, cytokinins, and gibberellins, and reduced the production of ethylene, ABA, and phenols (Alharbi et al. 2022a). Therefore, the PGPR maintained the redox balance of the cell, resulting in the detoxification of H2O2, converting it to H2O and O2 (Omara et al. 2022). Furthermore, the decline in lipid peroxidation (MDA) and EL compared to untreated plots (CK) under saline conditions is an indication for alleviating the harmful effects of salinity-induced oxidative stress because of its potential to maintain the electron transport chain along with its considerable influence as a biostimulant in protecting the plant cells against oxidative stress through its direct or indirect effect on osmoregulation, protein stabilization, and antioxidants stability (Alharbi et al. 2022b). Electron leakage from the electron transport chain is a potential source of ROS generation. Plant cells have evolved mechanisms to minimize electron leakage, such as the presence of alternative electron sinks and regulatory processes that help dissipate excess electrons safely. The proper functioning of the electron transport chain is crucial for maintaining a balance between ROS generation and antioxidant defense in plant cells. When the electron transport chain is functioning optimally, it can maintain a controlled production of ROS, which is essential for normal cellular signaling and defense responses without causing excessive oxidative stress (Döring and Lüthje 1996).
Antioxidant activity, reduced Na+ uptake, and ion toxicity
The foliar application of ZnO-NPs promoted the biosynthesis of photosynthetic pigments, i.e., chl a, chl b, and carotenoids, physiological performance, and maintenance of better biochemical attributes due to its role in regulating the activities of antioxidant enzymes (e.g., CAT, POX, and SOD) (Azam et al. 2022). Therefore, it fastened the conversion of toxic H2O2 into non-toxic compounds (i.e., H2O and O2), protecting cell membrane from damage under salt stress, which increased nutrient uptake (N, P, and K) as indicated by higher NPK contents in wheat seeds due to the application of ZnO-NPs (Kheir et al. 2019). The integrated application of PGPR and ZnO-NPs was further persuasive in alleviating oxidative stress than the single application due to better conversion of ROS into less or non-toxic compounds under salt stress. In the present study, the application of PGPR+ZnO-NPs lowered the Na+ accumulation in the cell cytosol while increasing K+, leading to high K+/Na+ ratio and speeding up saline stress tolerance (Hafez et al. 2021). Also, PGPR were reported to reduce uptake of Na+ ion while increase K+ absorption by plants through altering their concentrations in soil solution and consequently cause physiological balance in the leaf tissues (Shahid et al. 2020). PGPR can help decrease the Na+ in the soil solution through several mechanisms that promote Na+ removal or reduce Na+ uptake by plants. For example, certain PGPR have the ability to absorb or sequester Na+ within their cells. This process effectively removes Na+ from the soil solution and decreases its availability for uptake by plants. Some PGPR produce organic acids as metabolic byproducts. These organic acids can chelate with Na+ in the soil, forming stable complexes that are less available for plant uptake. As a result, the overall concentration of available Na+ in the soil solution decreases. PGPR can secrete extracellular polysaccharides, which improve soil aggregation and structure. Enhanced soil structure can reduce Na+ buildup and improve water drainage, preventing the accumulation of excessive Na+ in the root zone (Ullah and Bano 2019). On the other hand, PGPR can increase K+ concentration in the soil solution through various mechanisms. Some PGPR have the ability to solubilize fixed K in the soil, converting insoluble K minerals into soluble forms. These bacteria produce organic acids and enzymes that break down the mineral structures, releasing K+ into the soil solution. Certain PGPR can produce specific enzymes that facilitate the release of K+ from organic matter in the soil. This mobilization of K+ from organic sources makes it more available to plants for uptake. Some PGPR can act as biofertilizers, synthesizing organic compounds or siderophores that chelate K+, making it more accessible to plants by preventing its fixation or leaching. PGPR can compete with other soil microorganisms for K+ uptake. By outcompeting other microorganisms for this essential nutrient, PGPR ensure a greater portion of the available K+ is available to plants (Berde et al. 2021). The effect of the dual application of PGPR and ZnO-NPs could be attributed to the direct provision of PGPR to the root and further supply from the foliar spraying by ZnO-NPs. The stimulative impact of PGPR and ZnO-NPs jointly on membrane permeability integrity has been deemed reliable for improved plant nutrition (Yasmin et al. 2021). Seed inoculation with PGPR could enhance yield-related attributes like the number of grains per spike and 1000-grain weight (g) via augmenting cell division and enlargement (Abd El-Mageed et al. 2022) due to augmented nutrient and water absorption. The PGPR application as a growth promoter could deliver important metabolites, like phytohormones (e.g., IAA, auxins, and gibberellins), organic acid, and ACC deaminase, which finally enhance plant growth and crop yield. Our data are following those reported earlier by (Ahemad and Kibret 2014; Ahmad et al. 2022).
Enhanced nutrient uptake, ion balance, and wheat yield
The combined application showed higher enhancement in yield-related traits, yield, and NPK of wheat compared to the sole application (Danish and Zafar-ul-Hye 2019). The PGPR application could enhance nutrient uptake (NPK) from the soil solution due to discarding Na+ ions out of the soil solution, resulting in released auxin and cytokinin hormones and increased soil enzymes (Hidri et al. 2022). Furthermore, foliar application of ZnO-NPs could increase K+ ions in the leaves, which have a potential role as a transporter of nutrients and water. Increasing K+ content in the leaves increased the transportation of macro-elements (NPK) from leaves to sink, resulting in increased grain filling and sink size (Ahmed et al. 2023). So, the dual application of PGPR and ZnO-NPs revealed the highest NPK contents in wheat grains under salinity stress. Our examination exhibited that the PGPR+ZnO-NPs application to salt-stressed wheat plants mitigated the detrimental effects of irrigation with saline water by enhancing leaf water content, physiological, and biochemical attributes accompanied by enhancing antioxidant enzyme activity and, hence, a higher increase in yield-related traits and nutrient content.
Conclusions
The present study revealed the detrimental impacts of soil salinity on the growth and productivity of wheat plants. Moreover, irrigating plants grown in such soil with low-quality water (saline water) would magnify the problem because of the increased oxidative damage and nutritional imbalance. Nevertheless, the exogenous application of combined PGPR and ZnO-NPs significantly mitigated these detrimental impacts, enhancing plant growth and productivity via lowering ion toxicity, and oxidant levels, while increasing the activity of antioxidant enzymes and the efficiency of the photosynthetic machinery. Despite single application of PGPR or ZnO-NPs brought considerable positive alterations in wheat growth under salinity stress, the integrated application of PGPR+ZnO-NPs significantly increased the wheat grain yield, NPK uptake, Zn content in wheat straw and grains, RWC of wheat leaves, and activity of soil urease and dehydrogenase enzymes as well as it significantly reduced the contents of MDA and proline. Dual application of PGPR and different essential/ beneficial elements, particularly in their tiny sizes, would bring multiple benefits for agricultural sustainability, particularly in such harsh environments. However, further research is needed to affirm these findings on a large scale.
References
Abd El-Mageed TA, Abd El-Mageed SA, El-Saadony MT, Abdelaziz S, Abdou NM (2022) Plant growth-promoting Rhizobacteria improve growth, morph-physiological responses, water productivity, and yield of Rice plants under full and deficit drip irrigation. Rice 15:16. https://doi.org/10.1186/s12284-022-00564-6
Adil M, Bashir S, Bashir S, Aslam Z, Ahmad N, Younas T, Asghar RMA, Alkahtani J, Dwiningsih Y, Elshikh MS (2022) Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front Plant Sci 13:932861. https://doi.org/10.3389/fpls.2022.932861
Adrees M, Khan ZS, Hafeez M, Rizwan M, Hussain K, Asrar M, Alyemeni MN, Wijaya L, Ali S (2021) Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous cd and water deficient stress. Ecotoxicol Environ Saf 208:111627. https://doi.org/10.1016/j.ecoenv.2020.111627
Aebi H (1984) Catalase in vitro. In: Methods in Enzymology. Elsevier, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science 26:1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Ahmad MT, Asghar HN, Saleem M, Khan MY, Zahir ZA (2015) Synergistic effect of rhizobia and biochar on growth and physiology of maize. Agron J 107:2327–2334. https://doi.org/10.2134/agronj15.0212
Ahmad HM, Fiaz S, Hafeez S, Zahra S, Shah AN, Gul B, Aziz O, Mahmood-Ur-Rahman, Fakhar A, Rafique M, Chen Y, Yang SH, Wang X (2022) Plant growth-promoting Rhizobacteria eliminate the effect of drought stress in plants: a review. Front Plant Sci 13:875774. https://doi.org/10.3389/fpls.2022.875774
Ahmed R, Uddin MK, Quddus MA, Samad MYA, Hossain MAM, Haque ANA (2023) Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 9:162. https://doi.org/10.3390/horticulturae9020162
Al Jabri H, Saleem MH, Rizwan M, Hussain I, Usman K, Alsafran M (2022) Zinc oxide nanoparticles and their biosynthesis: overview. Life 12:594. https://doi.org/10.3390/life12040594
Alharbi K, Hafez E, Omara AE-D, Awadalla A, Nehela Y (2022a) Plant growth promoting Rhizobacteria and silica nanoparticles stimulate sugar beet resilience to irrigation with saline water in salt-affected soils. Plants 11:3117. https://doi.org/10.3390/plants11223117
Alharbi K, Rashwan E, Mohamed HH, Awadalla A, Omara AE-D, Hafez EM, Alshaal T (2022b) Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 11:2026. https://doi.org/10.3390/plants11152026
AOAC (ed) (2016) Official methods of analysis of AOAC international, 20th edn. AOAC International, Gaithersburg
Atlas RM, Atlas RM (2004) Handbook of microbiological media, 0th edn. CRC Press. https://doi.org/10.1201/9781420039726
Awan S, Shahzadi K, Javad S, Tariq A, Ahmad A, Ilyas S (2021) A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J Saudi Soc Agric Sci 20:18–24. https://doi.org/10.1016/j.jssas.2020.10.003
Azam M, Bhatti HN, Khan A, Zafar L, Iqbal M (2022) Zinc oxide nano-fertilizer application (foliar and soil) effect on the growth, photosynthetic pigments and antioxidant system of maize cultivar. Biocatalysis and Agricultural Biotechnology 42:102343. https://doi.org/10.1016/j.bcab.2022.102343
Azmat A, Tanveer Y, Yasmin H, Hassan MN, Shahzad A, Reddy M, Ahmad A (2022) Coactive role of zinc oxide nanoparticles and plant growth promoting rhizobacteria for mitigation of synchronized effects of heat and drought stress in wheat plants. Chemosphere 297:133982. https://doi.org/10.1016/j.chemosphere.2022.133982
Bajji M, Kinet J-M, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70. https://doi.org/10.1023/A:1014732714549
Barnawal D, Singh R, Singh RP (2019) Role of plant growth promoting rhizobacteria in drought tolerance. In: PGPR Amelioration in Sustainable Agriculture. Elsevier, pp. 107–128. https://doi.org/10.1016/B978-0-12-815879-1.00006-9
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Berde CV, Gawde SS, Berde VB (2021) Potassium Solubilization: mechanism and functional impact on plant growth. In: Yadav AN (ed) Soil microbiomes for sustainable agriculture, sustainable development and biodiversity. Springer International Publishing, Cham, pp 133–148. https://doi.org/10.1007/978-3-030-73507-4_5
Cappelli A, Cini E (2021) Challenges and opportunities in wheat flour, pasta, bread, and bakery product production chains: a systematic review of innovations and improvement strategies to increase sustainability, productivity, and product quality. Sustainability 13:2608. https://doi.org/10.3390/su13052608
Danish S, Zafar-ul-Hye M (2019) Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci Rep 9:5999. https://doi.org/10.1038/s41598-019-42374-9
Döbereiner J, Day JM (1976) Associative Symbiosis in tropical grasses: characterization of microorganisms and dinitrogen fixing sites in Sysposium on nitrogen fixation. Washington State University Press
Döring O, Lüthje S (1996) Molecular components and biochemistry of electron transport in plant plasma membranes (review). Mol Membr Biol 13:127–142. https://doi.org/10.3109/09687689609160589
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570. https://doi.org/10.1021/jf00021a018
El-Saadony MT, Saad AM, Soliman SM, Salem HM, Desoky E-SM, Babalghith AO, El-Tahan AM, Ibrahim OM, Ebrahim AAM, Abd El-Mageed TA, Elrys AS, Elbadawi AA, El-Tarabily KA, AbuQamar SF (2022) Role of nanoparticles in enhancing crop tolerance to abiotic stress: a comprehensive review. Front Plant Sci 13:946717. https://doi.org/10.3389/fpls.2022.946717
El-Zohri M, Al-Wadaani NA, Bafeel SO (2021) Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 10:2400. https://doi.org/10.3390/plants10112400
Erenstein O, Jaleta M, Mottaleb KA, Sonder K, Donovan J, Braun H-J (2022) Global trends in wheat production, consumption and trade. In: Reynolds MP, Braun H-J (eds) Wheat improvement. Springer International Publishing, Cham, pp 47–66. https://doi.org/10.1007/978-3-030-90673-3_4
Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, Yu F (2021) Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 161:122–130. https://doi.org/10.1016/j.plaphy.2021.02.002
FAO (2016) The State of Food and Agriculture 2016: Climate Change, Agriculture and Food Security. http://www.fao.org/3/a-i6030e.pdf. Accessed 13 Mar 2023
Figueiredo MDVB, Bonifacio A, Rodrigues AC, De Araujo FF (2016) Plant growth-promoting rhizobacteria: key mechanisms of action. In: Choudhary DK, Varma A (eds) Microbial-mediated induced systemic resistance in plants. Springer Singapore, Singapore, pp 23–37. https://doi.org/10.1007/978-981-10-0388-2_3
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37:604. https://doi.org/10.1071/FP09269
Gerhardt KE, MacNeill GJ, Gerwing PD, Greenberg BM (2017) Phytoremediation of salt-impacted soils and use of plant growth-promoting rhizobacteria (PGPR) to enhance phytoremediation. In: Ansari AA, Gill SS, Gill R, Lanza G, Newman L (eds) Phytoremediation. Springer International Publishing, Cham, pp 19–51. https://doi.org/10.1007/978-3-319-52381-1_2
Ghorai AK, Patsa R, Jash S, Dutta S (2021) Microbial secondary metabolites and their role in stress management of plants, in: Biocontrol Agents and Secondary Metabolites. Elsevier, pp. 283–319. https://doi.org/10.1016/B978-0-12-822919-4.00012-0
Grote U, Fasse A, Nguyen TT, Erenstein O (2021) Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front Sustain Food Syst 4:617009. https://doi.org/10.3389/fsufs.2020.617009
Hafez EM, Osman HS, Gowayed SM, Okasha SA, Omara AE-D, Sami R, Abd El-Monem AM, Abd El-Razek UA (2021) Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting Rhizobacteria and silica nanoparticles. Agronomy 11:676. https://doi.org/10.3390/agronomy11040676
Helaly MN, El-Hoseiny H, El-Sheery NI, Rastogi A, Kalaji HM (2017) Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol Biochem 118:31–44. https://doi.org/10.1016/j.plaphy.2017.05.021
Hidri R, Mahmoud OM-B, Zorrig W, Mahmoudi H, Smaoui A, Abdelly C, Azcon R, Debez A (2022) Plant growth-promoting Rhizobacteria alleviate high salinity impact on the halophyte Suaeda fruticosa by modulating antioxidant defense and soil biological activity. Front Plant Sci 13:821475. https://doi.org/10.3389/fpls.2022.821475
Kheir AMS, Abouelsoud HM, Hafez EM, Ali OAM (2019) Integrated effect of nano-Zn, nano-Si, and drainage using crop straw–filled ditches on saline sodic soil properties and rice productivity. Arab J Geosci 12:471. https://doi.org/10.1007/s12517-019-4653-0
Kumawat KC, Sharma B, Nagpal S, Kumar A, Tiwari S, Nair RM (2023) Plant growth-promoting rhizobacteria: salt stress alleviators to improve crop productivity for sustainable agriculture development. Front Plant Sci 13:1101862. https://doi.org/10.3389/fpls.2022.1101862
Lichtenthaler, H.K., 1987. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes, in: Methods in Enzymology. Elsevier, pp. 350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Liu L, Nian H, Lian T (2022) Plants and rhizospheric environment: affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol Biochem 185:91–100. https://doi.org/10.1016/j.plaphy.2022.05.032
McGeorge WT (1954) Diagnosis and improvement of saline and alkaline soils. Soil Sci Soc Am J 18:348. https://doi.org/10.2136/sssaj1954.03615995001800030032x
Mokrani S, Nabti E, Cruz C (2020) Current advances in plant growth promoting Bacteria alleviating salt stress for sustainable agriculture. Appl Sci 10:7025. https://doi.org/10.3390/app10207025
Munns R (2002) Comparative physiology of salt and water stress: comparative physiology of salt and water stress. Plant Cell Environ 25:239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043. https://doi.org/10.1093/jxb/erj100
Neshat M, Abbasi A, Hosseinzadeh A, Sarikhani MR, Dadashi Chavan D, Rasoulnia A (2022) Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol Mol Biol Plants 28:347–361. https://doi.org/10.1007/s12298-022-01128-0
Nivetha N, Lavanya AK, Vikram KV, Asha AD, Sruthi KS, Bandeppa S, Annapurna K, Paul S (2021) PGPR-mediated regulation of antioxidants: prospects for abiotic stress management in plants. In: Singh HB, Vaishnav A, Sayyed RZ (eds) Antioxidants in plant-microbe interaction. Springer Singapore, Singapore, pp 471–497. https://doi.org/10.1007/978-981-16-1350-0_23
Omara AE-D, Hafez EM, Osman HS, Rashwan E, El-Said MAA, Alharbi K, Abd El-Moneim D, Gowayed SM (2022) Collaborative impact of compost and beneficial Rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 11:877. https://doi.org/10.3390/plants11070877
Paliwal A, Verma A, Tiwari H, Singh MK, Gour JK, Nigam AK, Kumar R, Sinha VB (2021) Effect and importance of compatible solutes in plant growth promotion under different stress conditions. In: Wani SH, Gangola MP, Ramadoss BR (eds) Compatible solutes engineering for crop plants facing climate change. Springer International Publishing, Cham, pp 223–239. https://doi.org/10.1007/978-3-030-80674-3_10
Pereira SIA, Abreu D, Moreira H, Vega A, Castro PML (2020) Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 6:e05106. https://doi.org/10.1016/j.heliyon.2020.e05106
Phale PS, Sharma A, Gautam K (2019) Microbial degradation of xenobiotics like aromatic pollutants from the terrestrial environments, in: Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology. Elsevier, pp. 259–278. https://doi.org/10.1016/B978-0-12-816189-0.00011-1
Sadigov R (2022) Rapid growth of the world population and its socioeconomic results. Sci World J 2022:1–8. https://doi.org/10.1155/2022/8110229
Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, Gómez C, Mattson N, Nasim W, Garcia-Sanchez F (2020) Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 10:938. https://doi.org/10.3390/agronomy10070938
Sheteiwy MS, Shaghaleh H, Hamoud YA, Holford P, Shao H, Qi W, Hashmi MZ, Wu T (2021) Zinc oxide nanoparticles: potential effects on soil properties, crop production, food processing, and food quality. Environ Sci Pollut Res 28:36942–36966. https://doi.org/10.1007/s11356-021-14542-w
Singh RP, Handa R, Manchanda G (2021) Nanoparticles in sustainable agriculture: an emerging opportunity. J Control Release 329:1234–1248. https://doi.org/10.1016/j.jconrel.2020.10.051
Sparks DL, Page AL, Helmke PA, Loeppert RH (1996) Methods of Soil Analysis Part 3—Chemical Methods. Soil Science Society of America Book Series 5.3. Madison, USA
Svane S, Sigurdarson JJ, Finkenwirth F, Eitinger T, Karring H (2020) Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci Rep 10:8503. https://doi.org/10.1038/s41598-020-65107-9
Tsukanova KA, Сhеbоtаr VК, Meyer JJM, Bibikova TN (2017) Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102. https://doi.org/10.1016/j.sajb.2017.07.007
Ullah A, Bano A (2019) Role of PGPR in the reclamation and revegetation of saline land. Pak J Bot 51. https://doi.org/10.30848/PJB2019-1(43)
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vetter JL, Steinberg MP, Nelson AI (1958) Enzyme assay, quantitative determination of peroxidase in sweet corn. J Agric Food Chem 6:39–41. https://doi.org/10.1021/jf60083a006
Wheeler T, Von Braun J (2013) Climate change impacts on global food security. Science 341:508–513. https://doi.org/10.1126/science.1239402
Yasmin H, Mazher J, Azmat A, Nosheen A, Naz R, Hassan MN, Noureldeen A, Ahmad P (2021) Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol Environ Saf 218:112262. https://doi.org/10.1016/j.ecoenv.2021.112262
Yoshida Y, Marubodee R, Ogiso-Tanaka E, Iseki K, Isemura T, Takahashi Y, Muto C, Naito K, Kaga A, Okuno K, Ehara H, Tomooka N (2016) Salt tolerance in wild relatives of adzuki bean, Vigna angularis (Willd.) Ohwi et Ohashi. Genet Resour Crop Evol 63:627–637. https://doi.org/10.1007/s10722-015-0272-0
Zahoor S, Naz R, Keyani R, Roberts TH, Hassan MN, Yasmin H, Nosheen A, Farman S (2022) Rhizosphere bacteria associated with Chenopodium quinoa promote resistance to Alternaria alternata in tomato. Sci Rep 12:19027. https://doi.org/10.1038/s41598-022-21857-2
Zhang H, Wang R, Chen Z, Cui P, Lu H, Yang Y, Zhang H (2021) The effect of zinc oxide nanoparticles for enhancing Rice (Oryza sativa L.) yield and quality. Agriculture 11:1247. https://doi.org/10.3390/agriculture11121247
Zou P, Li K, Liu S, He X, Zhang X, Xing R, Li P (2016) Effect of sulfated Chitooligosaccharides on wheat seedlings (Triticum aestivum L.) under salt stress. J Agric Food Chem 64:2815–2821. https://doi.org/10.1021/acs.jafc.5b05624
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R188), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the support provided by the following institutions; Faculty of Agriculture, Kafrelsheikh University, Egypt; Soils, Water and Environment Research Institute (SWERI), Agriculture Research Center (ARC), Egypt. This work has been implemented with the TKP2021-EGA-20 support provided from the National Research, Development and Innovation Fund of Hungary.
Funding
Open access funding provided by University of Debrecen. This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R188), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Khadiga Alharbi, Emad M. Hafez, Alaa El-Dein Omara, Emadelden Rashwan, and Tarek Alshaal. The first draft of the manuscript was written by Emad M. Hafez, and Tarek Alshaal and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Gustavo Gabriel Striker.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alharbi, K., Hafez, E.M., Omara, A.ED. et al. Zinc oxide nanoparticles and PGPR strengthen salinity tolerance and productivity of wheat irrigated with saline water in sodic-saline soil. Plant Soil 493, 475–495 (2023). https://doi.org/10.1007/s11104-023-06245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06245-7