Abstract
Background and aim
The use of amendments to immobilize metals in polluted soils is a widely accepted remediation approach, and in the framework of the circular economy, amendments produced from mining and/or biomass waste have gained relevance. However, the application of such amendments can also mobilize metalloids. Here we propose the combination of nanoscale zero-valent iron nanoparticles (nZVI) with dunite (mining waste) and compost for the remediation and restoration of soil affected by high concentrations of As and metals.
Methods
To this end, we treated pots containing the polluted soil with combinations of dunite, compost, and nZVI for 75 days. In addition, Sinapis alba was used to evaluate the effects of the amendments on pollutant accumulation in the plant. The mobility of the pollutants was monitored through TCLP extraction and by sampling pore water. Furthermore, pH, available P, and cation exchange capacity (CEC) were also determined.
Results
Dunite application led to the immobilization of metals, and supplied Mg, thus improving CEC. On the other hand, compost increased nutrient content, and also promoted plant growth. However, this amendment caused a dramatic increase in As accumulation in the plants. Finally, the application of nZVI in combination with the other two amendments was found to be the most appropriate strategy since it not only prevented As mobilization and accumulation but also added nutrients to the soil, thus promoting plant growth.
Conclusion
The combination of nZVI with dunite mining waste and compost proved effective for the remediation of soil simultaneously polluted by As and metals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industrial development in urban and periurban areas over recent decades has left numerous abandoned areas (brownfields) that have still not been recovered (Hammond et al. 2021; Wcisło et al. 2016). The most notable types of degraded brownfields found in urban areas are contaminated by metal(loid)s (Niu and Lin 2021). In this regard, several technologies, such as phytoremediation, soil washing, and bioremediation, are available to tackle this issue (Aparicio et al. 2022; Rayu et al. 2012). In this context, a new emerging concept, named nature-based solutions (NBS), offers great potential for the remediation of contaminated land and brownfield redevelopment (Song et al. 2019; Wickenberg et al. 2021). Within NBS, amendments of either organic or inorganic origin or a combination of the two are already being used to stabilize soil. The combination of NBS with phytoremediation techniques has also been addressed (Kisser et al. 2020; Martínez-Hernández et al. 2020; Song et al. 2019).
Metal(loid) immobilization is a soil remediation strategy of growing interest owing to its fast and easy application and its commercial viability (Wang et al. 2021; Zhang et al. 2021). This technique aims to lower the mobility of metal(loid)s in soil, thereby reducing their availability and avoiding uptake by plants (Palansooriya et al. 2020; Wu et al. 2021). Soil amendments have proven effective as immobilizing agents and soil ameliorants. However, no single amendment is effective in all types of degraded soils (Park et al. 2011). Evaluation of the physicochemical properties of both the soil and amendments is crucial for selecting the most appropriate combination to simultaneously immobilize metals and metalloids in brownfields affected by multielement contamination.
The possibility of turning an organic or inorganic subproduct into a valuable soil amendment fully complies with the principles of the circular economy (Breure et al. 2018; Hou and Al-Tabbaa 2014). On the one hand, huge amounts of inorganic subproducts with no economic value are generated in mining operations, and their re-utilization is a universal challenge (Agboola et al. 2020). This mining waste is frequently non-hazardous and its properties are favourable for other uses, such as the recovery of degraded soils (García et al. 2004; Ray et al. 2021). Dunite mining waste has been successfully used as a soil amendment to partially immobilize some metals and enhance soil properties (Baragaño et al. 2019). On the other hand, organic amendments like compost, a typical fertilizer used mainly in agriculture but also for contaminated soil recovery (Forján et al. 2018a, b), increase nutrient and organic matter content and improve the physical properties of the soil (Beesley et al. 2014; Mudhoo et al. 2020). However, As and other metalloids can be mobilized (Beesley et al. 2010); moreover, depending on the raw material used for producing the compost, it commonly contains inappropriate amounts of metal(loid)s (Baragaño et al. 2021a).
To deal with the relevance and implications of soil pollution (Lal et al. 2021), innovative technologies, such as nanoremediation, have been explored over the last 10 years. In this regard, the application of zero-valent iron nanoparticles (nZVI) has gained special interest (Li and Liu 2021; Stefaniuk et al. 2016; Vítková et al. 2018). Comprised of a highly reactive material with a large specific surface that can react with pollutants such as As, these nanoparticles show high efficiency (Baragaño et al. 2020a). However, nZVI do not provide nutrients to soil and they are not fully effective with some metals. Therefore, it is appropriate to combine nZVI with organic and inorganic amendments that counteract these issues (Baragaño et al. 2020b).
Following the preceding considerations, here we tested the capacity of nZVI to mitigate As mobilization and accumulation when used with a mixture of inorganic and organic amendments (dunite mining waste and compost) to remediate soil affected by multi-element contamination. To this end, Sinapis alba was used as an indicator plant in an experimental design with a soil representative of a brownfield affected by As and metal pollution.
Materials and methods
Polluted soil and amendments
The polluted soil was taken from an industrial area located in Northern Spain that operated for more than fifty years until its closure in 1997 (Baragaño et al. 2020c; Gallego et al. 2016). After an exhaustive study of the distribution of pollutants in the study area (data not shown due to confidentiality), a composite sample of 20 kg of soil (labelled S) was taken using standard procedures to obtained a representative sample from the polluted soils found in the site. The physicochemical characterization of the polluted soil is summarized in Table 1.
The physicochemical characteristics of the inorganic (dunite mine waste) and organic (compost) amendments are described in Table 1. The dunite mining waste (D) was taken from the processing plant of David Mine, located in Galicia, Spain. The sample used in this study belongs to the finest fractions (<63 μm) generated during the mining process (Baragaño et al. 2019). The compost (C), provided by Piensos Lago S.L. (Asturias, Spain), was made from animal manure and plant debris.
In turn, commercial nZVI, namely NANOFER 25S, were supplied by NANOIRON s.r.o. (Czech Republic). According to the specifications, the suspension have a Fe(0) content of 14–18% and 2–6% of magnetite. The particles have an average diameter of 60 nm, a strongly alkaline suspension (pH 11–12), and an active surface area of 20 m2/g (additional details are available at www.nanoiron.cz).
Mineralogical analysis of dunite mining waste
The mineralogical composition of the dunite mining waste was assessed by powder x-ray diffraction (PXRD) on a PANalytical X’Pert Pro MPD diffractometer with Cu Kα1 radiation (1.540598 Å). After determining the position of Bragg peaks over the range of 2θ = 5–80°, the minerals were identified using databases of the International Centre for Diffraction Data, ICDD PDF-2 catalog (ICDD 2003). Furthermore, the chemical composition of the dunite particles were also measured by scanning-electron microscopy and energy-dispersive X-ray spectroscopy (SEM-EDX) using a JEOL JSM-5600 Scanning Electron Microscope coupled to an Energy Dispersive X-ray analyzer (INCA Energy 200) to supplement the information acquired by XRD.
Greenhouse experiment
A total of 12 plant pots—3 per treatment—were placed randomly in a greenhouse for 75 days. All pots, which contained 1 kg of total mass, comprised 80% of contaminated soil and 20% of amendment, except the pots in the control treatment (S), which contained only contaminated soil. These proportions were chosen based on previous studies where organic and inorganic amendments were applied (Baragaño et al. 2019; Baragaño et al. 2021b). Specifically, the application of the high dose of the dunite mining waste is justified given the nature of the residue, a material with no economic value for the mining company. For the dunite amendment (SD) experiment, the proportion of dunite accounted for 20% of the total, while in the dunite-compost mixture (SDC), each amendment accounted for 10% of the total. Finally, 10% of both dunite and compost plus 2% (w/w) of nZVI suspension was used for the SDCN treatment, which means 0.4% (w/w) of nZVI powder (Baragaño et al. 2020a; Forján et al. 2021). Sinapis alba was grown to confer stability to the soil and to evaluate metal(loid) uptake by the plant as it is a usual indicator plant for remediation proposals (Jedynak and Kowalska 2011; Vaněk et al. 2010). Before planting the S. alba individuals, the polluted soil (S) and the treated soils (SD, SDC, and SDCN) were pre-incubated in the pots for 7 days by watering. During this time, S. alba seeds were germinated in a substrate, and then, small plants were transplanted to the pots. The pots were watered to field capacity throughout the experiment, and the greenhouse was maintained at an average temperature of 13 ± 4 °C and 15/9 h (day/night cycle).
At day 0, 7, 15, 45, and 75, pore water samples were collected to determine As and metal (Cd, Cu, Pb, and Zn) by applying suction to rhizon samplers (Rhizosphere research products, Wageningen, Netherlands) previously collocated in the above part of the pots. The element concentrations were determined by ICP-OES (Optima 4300 DV; Perkin-Elmer). Soil and plants were removed and processed for further analyses at the end of the experiment.
Soil analyses
For analytical procedures, soil samples were air-dried, sieved at 2 mm, and homogenized. The pH was measured using a pH electrode in a 1:2.5 water/soil proportion (Porta et al. 1986). The total carbon (TC) and total nitrogen (TN) concentrations of the samples were determined in a LECO CN-2000 module. TCLP extraction (USEPA 1992) was used to assess the concentration of extractable metal(loid)s. Pseudo-total metal(loid) concentrations were determined by ICP-OES after acid digestion in a microwave oven (Milestone ETHOS 1, Italy) using aqua regia. Blanks were measured each 10 samples and analytical duplicates were also used. In addition, the analytical quality control was conducted using the certified reference material NIST SRM 2711a, Soil Montana II (characterization in Table S1). Exchangeable cation concentrations (Ca2+, K+, Mg2+, Na+, Al3+) were extracted using 0.1 M BaCl2 (Hendershot and Duquette 1986) and determined by ICP-OES. The Cation Exchange Capacity (CEC) was calculated following Houba et al. (2000). Finally, available phosphorus (P) concentration was determined by the Mehlich 3 method (Mehlich 1984).
For determining As speciation in soil samples, 0.1 g of soil and 15 mL of the extracting agent (1 M H3PO4 + 0.1 M ascorbic acid) were placed in a microwave vessel and digested (Multiwave 3000, Anton Paar GmbH) at 60 W for 10 min (Garcia-Manyes et al. 2002). The extracts were consequently diluted and filtered (0.45 μm). The As species were separated in a 4.6 mm × 150 mm As Separation Column (Agilent Technologies) fitted to a 1260 Infinity HPLC coupled to a 7700 ICP-MS (Agilent Technologies) using a mobile phase of 2 M PBS (Phosphate Buffered Saline)/0.2 M EDTA (pH = 6.0) at a flow of 1 mL/min.
Plant analyses
At the end of the experiment, all plants were harvested at the same stage of maturity (pre-flowering state). The aerial part and roots of each plant were separated and gently washed with deionised water (ultrasound-assisted washing was performed to remove remaining soil particles from roots). The height of the plant was measured, and the aerial part biomass was weighed. After oven-drying for 48 h at 80 °C, and cooling at room temperature, dry biomass was determined.
The translocation factor (TF), defined by Baker and Brooks (1989), was determined using the following equation:
where Cs is the metal(loid)s concentration in the plant shoots and Cr is the metal(loid)s concentration in the plant roots, both in mg/kg. A plant translocates metal(loid)s effectively from the roots to shoots when TF > 1.
The bioaccumulation factor (BAF) in the plants was also determined, which measures their efficiency to take up metal(loid)s from the soil, assessed by taking the available forms instead of the soil pseudo-total concentrations as a reference (Rodríguez-Vila et al. 2014), as expressed by the following equation:
where Cap is the metal(loid) concentration in the aerial part (mg/kg) and Cs is the extractable metal(loid) concentration in the soil (mg/kg).
The concentration of As, Cd, Cu, Pb, and Zn in the roots and the aerial part of the plant was analysed by ICP-OES after acid digestion using HNO3 in a microwave oven. In addition, based on the models of the Soil Fertility Capability Classification (SFCC) proposed by Sanchez et al. (1982), various factors (e factor, K factor, M factor, n factor, Ca/Mg ratio and TN content) related to plant production were determined and then compared with the critical values defined by the aforementioned authors.
Statistical analysis
All analytical determinations were performed in triplicate. The data obtained were treated using the SPSS program, version 27.0 for Windows. Analysis of variance (ANOVA) and test of homogeneity of variance were carried out. In the case of homogeneity, a post hoc least significant difference (LSD) test was carried out. If there was no homogeneity, Dunnett’s T3 test was performed. A correlated bivariate analysis was also performed by means of Pearson correlation.
Results
Dunite mining waste characterization
The diffraction pattern obtained by XRD (Fig. S1) showed that dunite mining waste is composed mainly of minerals from the serpentine group, such as lizardite (Ref. Code: 00-050-1625; (Mg,Al)3((Si,Fe)2O5)(OH)4), and also from the chlorite group, such as clinochlore (Ref. Code: 01-087-2496; Mg5Fe0.2Al2Si3O10(OH)8) and kammererite (Ref. Code: 00-012-0240; Mg5(Al,Cr)2Si3O10(OH)9). Accessory minerals as hornblende (Ref. Code: 01-071-1062; (K0.3Na0.6)(Ca2Mg0.3)(Mg3Fe2Al0.3Ti0.2)Al2Si6O22(OH)2), ankerite (Ref. Code: 01-079-1349; Ca(Mg0.27Fe0.68Mn0.05)(CO3)2) and dolomite (Ref. Code: 00-034-0517; Ca(Mg,Fe)(CO3)2) are presented in low concentrations. The complementary SEM analysis (Fig. 1) revealed the presence of elements such as Al, Ca, Fe, Mg and Si, some of the major elements presented in the composition of the identified minerals, in accordance with the XRD characterization. Regarding trace elements, only Cu and Zn were detected in low concentrations using this technique.
Pseudo-total concentrations and metal(loid) extractability
The polluted soil (Table 1) was notably contaminated with As, Cd, Cu, Pb, and Zn, although after the application of the amendments, As and metals concentrations were slightly decreased (Table 2), specially in case of dunite mining waste application.
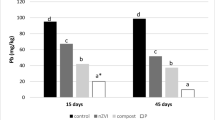
Figure 2 shows the extractable concentrations of As, Cd, Cu, Pb, and Zn as determined by TCLP extraction for each treatment at the end of the experiment. The use of dunite (SD) caused a significant reduction in the extractable concentration of Cd, Cu, Pb and Zn compared to the control (S), while the extractable concentration of As was very low and the difference between S and SD was almost negligible (Fig. 2). However, when compost was added to the mixture (SDC), the extractable concentrations of Cd, Cu, Pb, and Zn were significantly reduced compared to both the S and SD treatments (Fig. 2). In contrast, the extractable concentration of As rose significantly in the SDC treatment compared to S (Fig. 2). Of note, the use of nZVI caused a decrease in the availability of As when compared with the SDC treatment. However, the extractable concentration of As was significantly higher in the SDCN treatment compared to S. In turn, the SDCN treatment registered the lowest extractable concentration of Pb of all the treatments (Fig. 2). Finally, for Cd, Cu and Zn, the addition of nZVI did not cause a relevant variation regarding the SDC treatment.
Arsenic speciation in soils
The analysis of As speciation in all soil samples, polluted soil and treated soils, revealed the predominance of As(V), around 96% in all samples without significant differences, which is in accordance with the source of the pyrite ashes presented in the soils due to the pyrite roasting under oxidant conditions. The application of the amendments did not modify the As speciation in the soil.
Evolution of As and metals in pore water
Figure 2 also shows the evolution of the concentrations of As, Cd, Cu, Pb and Zn in pore water on day 0, 7, 15, 45, and 75. For the S and SD treatments, all metal(loid)s, except Zn, showed concentrations in pore water below the limit detection value (40, 5, 5 and 20 μg/L for As, Cd, Cu and Pb respectively). However, the concentration of Zn, decreased over time in these two treatments. In contrast, in the treatments with compost (SDC and SDCN), the concentration of this metal and also that of As and Cu increased over time. However, the SDCN treatment showed lower concentrations of the metal(loid)s than the SDC treatment.
Evolution of physico-chemical properties
At the end of the experiment (75 days), the soil in the distinct treatments had significantly different pH values, reaching the highest value for the SDCN treatment, followed by SDC, SD, and finally the control (S) (Table 2).
The significant increase in pH upon the addition of dunite and compost amendments compared to S was positively correlated with dunite contribution to Mg2+ (0.86, P < 0.01) and Ca2+ (0.92, P < 0.01), and compost contribution to K+ (0.89, P < 0.01) and Na+ (0.91, P < 0.01). The increase in pH in each treatment compared to S was also positively correlated with TC (0.84, P < 0.01) and N (0.90, P < 0.01) values.
In turn, the null N content in the SD treatment was enhanced by the addition of compost (Table 2). The highest TC content at the end of the experiment was observed in the SDC treatment. Although the addition of nZVI significantly reduced this value, the SDCN treatment attained significantly higher values compared to S and SD (Table 2). TC and N contents were positively correlated (0.94 and 0.93 respectively, P < 0.01) with the biomass collected, an outcome explained by the importance of these nutrients for plant growth (Schjønning et al. 2004).
The S and SD treatments revealed the lowest available P content, whereas the highest value for this parameter was achieved in the SDC treatment due to the compost addition (very rich in P as shown in Table 1). However, the aplication of nZVI in the SDCN treatment significantly reduced P availability (Table 2).
Monitoring cation exchange capacity
CEC values increased for all treatments with respect to S (Table 2). Indeed, the addition of dunite mining waste increased the content of Mg2+, which implied a significant increase in CEC. Furthermore, the dissolution of Mg bearing phases (see mineralogical characterization), and therefore the consumption of protons during the hydrolysis, resulted in an increase on the soil pH. The use of compost as amendment (SDC and SDCN) caused a dramatic increase in CEC, which reached its highest value in the SDC treatment. However, the addition of nZVI to the compost-dunite (SDCN) did not significantly reduce CEC values.
Plant production and growth
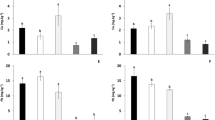
Figure 3 shows the fresh biomass and the length of the aerial part and root of Sinapis alba after 75 days of soil treatment. Significant differences were obtained regarding the amount of fresh biomass harvested (P < 0.05); the highest amount was collected in the SDC treatment, followed by SDCN, SD, and finally S (Fig. 3). Moreover, the length of the aerial part increased after the addition of each amendment. Regarding root length, the addition of each amendment also enhanced this parameter, except for the SDCN treatment, which caused an increase compared to S, but a decrease compared to the SD and SDC treatments.
Metal(loid) accumulation in Sinapis alba
Table 3 shows the concentrations of metal(loid)s in roots and aerial parts of the plants for each treatment. Generally, Pb and Zn were the metals with the greatest accumulation in the plant, followed by Cu and As.
The accumulation of Zn in the aerial part is higher in the plants growing in the polluted soil than in the treated soils. However, the application of compost in the SDC treatment revealed the highest accumulation of this metal in the roots. On the other hand, the application of dunite mining waste and nZVI decrease the Zn concentration in the roots of the plants, especially the addition of the nanoparticles.
Regarding the Pb concentrations in the plant system, it is not possible to observe differences in the aerial part of the plants between soil treatments, although Pb accumulation in roots is higher in the plants from the polluted soil than in the soil treatments. The application of dunite mining waste decreased the Pb concentrations in roots, and the addition of compost drastically decreased the accumulation of this metal. However, although roots of the plants growing in the nZVI-treated soil shown lower Pb concentration than roots from the polluted soil, higher concentrations are found in comparison with the dunite mining waste and compost application.
The application of compost to the soil drastically increases the Cu concentration in the aerial part of S. alba, although the combination with nZVI mitigates the Cu accumulation provoked by the compost. However, the Cu concentration in the roots of the plants growing in the SDC treatment revealed the lowest values.
The accumulation of As in the plants growing in the polluted soil is higher in the roots than in the aerial part, although the application of the dunite mining waste decreased the As accumulation in the roots. On the other hand, compost addition increased the As accumulation in the aerial part. However, the application of nZVI mitigates As accumulation in the aerial part, and also in the roots in comparison with the plants from the untreated polluted soil.
Finally, the accumulation of Cd in the aerial part and roots is very low compared to the other pollutants, and no differences were found between soil treatments.
Discussion
Initially, the polluted soil was notably contaminated with As, Cd, Cu, Pb, and Zn, as a result of the presence of pyrite ash at the site (Gallego et al. 2016; Oliveira et al. 2012). After the application of the dunite mining waste, a dilution effect (20% of dunite with very low metal and metalloid content was added) implied a reduction of total contents of the referred pollutants. According to the scientific literature, only elements such as Cr or Ni may be abundant in the ultramafic geological environment of dunite mines (Vithanage et al. 2019), although the mining activity in the site avoid the dunite production in areas slightly enriched in these elements. In fact, the chemical analysis using SEM (Fig. 1) shown not significant concentrations of these elements in the dunite mining waste, also supported by the mineralogical composition determined by XRD (Fig. S1). On the contrary, depending on the raw material used for compost production, the use of this amendment can lead to the introduction of some metal(loid)s into the soil (Beesley et al. 2010). In our case, the compost amendment contained a significant amount of Zn, but also As, Cu, and Pb (Table 1).
Despite the above, neither the addition of dunite mining waste nor the application of compost affected significantly the total concentrations of these metals (Table 2). Therefore, in all the experiments and for all the elements, the initial concentrations were observed to decrease. Given the high initial soil concentrations of these contaminants, this decrease was undoubtedly due to a dilution effect. These variations may be statistically significant in some cases but are not relevant in the framework of this study according to the extremely high concentrations of some metals in the soil.
On the other hand, after the application of the amendments it was revealed that the extractable concentrations of some metal(loid)s were modified. In conclusion, dunite (SD treatment) caused a significant reduction in the availability of Cu, Cd, Pb, and Zn and did not increase As availability compared to the control (S). These results are consistent with previous data obtained for the immobilization of metals after soil amendment with dunite (Baragaño et al. 2019). The decrease in the availability of Cd, Cu, Pb, and Zn was negatively correlated to the increase in pH (−0.94, −0.87, −0.97, and − 0.91, respectively; P < 0.01) caused by an enhanced Mg2+ content upon the addition of dunite mining waste, performed by the dissolution of Mg bearing phases. Therefore, the immobilization mechanism was metal precipitation, as it was shown in a previous work (Baragaño et al. 2019). Then, when compost was added as an organic amendment (SDC), the availability of Cu, Cd, Pb and Zn showed a greater reduction, but As availability was substantially increased. Baragaño et al. (2020b) also described these changes in the availability of As, Cu, Pb, and Zn after applying compost amendment. In this regard, this amendment increased organic matter, thereby enhancing negatively charged functional groups due to soil pH, and thus implying As mobilization. In the same context, Beesley et al. (2014) concluded that compost favors the potential for As leaching due to its effects on dissolved organic carbon and soluble P concentrations. Here we found positive correlations between the availability of As and P (0.96, P > 0.01), but negative correlations between Cd, Cu, Pb, Zn and P (−0.66, −0.51, −0.56, −0.56). These findings could be explained by the formation of insoluble metal orthophosphates from the combination of Cd, Cu, Pb, and Zn and the phosphate added by compost (Eighmy and Eusden 2004; Gong et al. 2018). Finally, the SDCN treatment entailed a reduction in the availability of all the metal(loid)s studied, except As, compared to the control. However, a comparison of the addition of nZVI (SDCN) with the SDC treatment revealed that the nanoparticles caused a significant depletion of the extractable concentrations of As and Pb and maintained those of the remaining metals low. When comparing As concentration in pore water before and after nZVI application (SDC and SDCN treatments), similar trend is shown, suggesting that the As immobilization due to nZVI is not dependent on ageing, but the As mobilization due to compost addition is increasing along time. The efficiency of nZVI to immobilize As through sorption onto the iron (hydro)oxide layer has been also reported (Baragaño et al. 2020d; O’Carroll et al. 2013; Vítková et al. 2018), although in our case, the interaction of nZVI with other elements, such as P provided from compost addition, revealed that the nanoparticles application was not fully efficient respecting As immobilization.
With regard to As, Cd, Cu, Pb and Zn in pore water, in general terms, the concentrations were low, excepting for As (up to 500 μg/L after the application of compost), and the variation of the results within treatments was not significant when compared with extraction in moderately acidic conditions, such as TCLP, as discussed above. In contrast, in the treatments with compost (SDC and SDCN), the concentration of this metal and also that of As and Cu increased over time. The increase in the concentrations of these metal(loid)s was directly correlated with pH (As 0.74, Cu 0.94, Zn 0.76, P < 0.01) and also with TC content (As 0.93, Cu 0.96, Zn 0.95, P < 0.01). The increase on the mobility of As and Cu are related to variation of soil pH and organic matter, as identified by other authors (Beesley et al. 2010), although the presence of these elements in the compost could not be negligible (Table 2).
Generally, the SDCN treatment showed lower concentrations of the metal(loid)s in pore water samples than the SDC treatment. This observation indicates that nZVI addition also mitigated the mobilization caused by the application of compost. Moreover, in these two treatments, three differentiated periods can be observed with respect to the metal(loid) concentrations in pore water. During the first 7 days, the release of the metal(loid)s increased dramatically. Then, from day 7 to 15, the concentration of the elements decreased abruptly. Finally, from day 16 to 75, the concentration of the elements gradually increased.
Regarding soil properties, the increase of pH, TN, TC and P was successfully achieved after the application of compost. However, it is remarkable that the increase of available P caused by the compost addition to the soil was mitigated by nZVI application (Table 2). The similar chemical behaviour of P and As explains the reduction in their availability upon the addition of nZVI to the dunite-compost mixture, as these two elements compete for binding sites on the iron (hydr)oxide layer (Baragaño et al. 2020a, b, c, d). If As would be dominantly in trivalent form, there will be limited sorption to hydrous ferric oxides, but in our case As(V) is the predominant form, suggesting As sorption onto these iron phases in the soil. However, the negatively charged functional groups of the organic matter cause a repulsive reaction between the soil surface and As anions (Arco-Lázaro et al. 2016; Woolson et al. 1973).
On the other hand, the enhanced CEC was also due to the increase in Ca2+, K+, Mg2+, and Na2+ concentration caused by the compost, as reported by other authors (Forján et al. 2017). However, although nZVI did not reduce CEC values, nZVI use may be related to the direct deposition of nanoparticles on roots, a process that blocks water and nutrient uptake (Ma et al. 2013; Stefaniuk et al. 2016). In fact, a positive correlation was found between the biomass collected and CEC (0.93, P < 0.01), as the latter is an indicator of nutrient availability and, therefore, plant growth capacity. In this sense, usual soil factors related to plant production are shown in Table 3. The control (S) revealed negative effects for all the factors analysed. However, after the addition of dunite (SD), most of these factors were favourable, except n factor and TN, due to the low Na and N content of the dunite mining waste (Table 1). Even after the addition of compost to the soil, the Na concentration in the soil was still below 15%, although closer values were found for the SDC (14.18%) and SDCN (13.91%) treatments.
The application of the mixture of amendments have shown changes not only in soil properties, but also on plant system. The biomass collected in the different treatments is congruent with the CEC values given in Table 2, and also with the limiting factors related to plant growth shown in Table 4. The application of factors related to plant production indicated that the fertility quality of the polluted soil is very poor, although after the application of the amendments the fertility of the soil is improved (Table 4). Of note is the difference in root length between treatments. In this regard, the application of compost and dunite (SDC) resulted in a notable increase in this parameter, while the addition of nZVI (SDCN) decreased it to the same value as plants growing in the control (S). The reduction in root length after the application of nZVI can not be explained by chemical parameters (As and metals concentrations in this part of the plant are lower in the SDCN treatment than in the polluted soil), but may be explained by physical parameters. Zhou et al. (2019) revealed that nZVI addition to the soil increases soil compaction, thus reducing root growth. However, the SDCN treatment showed the highest length of the aerial part, which could be attributed to the improvement of soil chemical properties (increase in pH, decrease in As availability).
Generally, Pb and Zn were the metals with the greatest accumulation in the plant. In fact, the metal(loid)s present in the soil at the highest concentration was also found in higher concentrations in the plants. Therefore, the highest accumulation was found for Pb and Zn, which was also reported in S. alba growing in other soils (Boshoff et al. 2014; Du et al. 2020).
Regarding the TF for the metal(loid)s, in the polluted soil only TF for Zn was below 1, indicating the capacity of this plant to translocate this element from roots to the aerial part (Table S3). However, the BAF values shown that the plant was not efficient at taking the metal(loid)s from the soil, although the highest values were found for Zn and Cd (0.24 and 0.19 respectively).
The application of the dunite mining waste decreased the Cu, Pb and Zn accumulation in the roots of the plant due to the decrease of the availability of these elements in the soil. Furthermore, the decrease on metals accumulation was also observed in the aerial part for Zn, although not for Cu and Pb. However, despite the lower metal(loid)s accumulation, the TF and BAF for each element revealed a slightly increase.
The addition of compost increased the accumulation of these metals in the plant, particularly for Zn in the roots and for As in the aerial part (Table 3), although this accumulation of metal(loid)s in S. alba growing in soils treated with compost differs from that found in other studies (Brunetti et al. 2012). According to pollutants availability, the increase in As uptake by the plant is related to the increase in labile forms of As seen in pore water, caused by the compost amendment (Clemente et al. 2010). The TF values for As, Cu and Pb drastically increased respecting to the untreated polluted soil, revealing that the capacity of the plants to translocate these elements was improved by the organic amendment application. Moreover, BAF values were also increased for As and Cu, revealing that the plants were able to extract both elements from the soil easily.
Finally, the accumulation of As and Zn was significant reduced after the addition of nZVI to the mixture (SDCN vs SDC), revealing that the decrease of the concentrations of both elements in pore water was useful to mitigate negative impacts on the plants. In accordance, the TF and BAF values for As decreased from 2.97 and 0.34 to 0.48 and 0.08 (SDC and SDCN treatments respectively). Therefore, the addition of nZVI considerably reduced the transfer of As from the soil to the plant as it was observed using similar plants by other authors (Vítková et al. 2018).
Conclusions
The application of mining and biomass waste for metal(loid) immobilization emerges as a promising low-cost approach within the context of the circular economy, although the interaction into the soil-plant system is not well-explored. In this regard, here we demonstrate the efficacy of nZVI-dunite mining waste-compost amendment to remediate and restore a metal(loid)s-polluted soil in combination with the plant S. alba.
Dunite mining waste was efficient at decreasing Cu, Pb, and Zn concentrations in pore water. Therefore, the accumulation of these elements in the roots of the plants also decreased. Moreover, Zn concentration in aerial part was decreased, although Cu and Pb concentrations were not modified. However, the low OM and N content, and low CEC values, caused mainly by deficient Ca2+ and Na+ content, resulted in poor biomass development.
On the other hand, the addition of compost to the dunite led to an increase in soil nutrient content, which in turn favoured plant growth. The dunite-compost amendment also proved a feasible strategy for metals immobilization in soil, although the addition of compost implied a slight increase in As concentration revealed by pore water analyses. However, the accumulation of metal(loid)s in the plant was severely increased, especially in case of As in the aerial part and Zn in the roots. Therefore, the plants were able to extract both elements easily from the soil.
Finally, the application of nZVI in combination with both amendments revealed that nanoparticles mitigated the mobilization of As in the pore water caused by the compost addition. Moreover, the accumulation of As and Zn was significant reduced after the addition of nZVI to the mixture with the soil.
In conclusion, our results reveal that the application of dunite mining waste is a feasible approach for reducing metals mobility and accumulation in plant, although compost is necessary for promoting plant growth under the risk of increasing pollutants accumulation. To solve it, the combination with nZVI results in the optimal option for mitigating pollutants accumulation in plant and stimulating plant growth simultaneously.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Agboola O, Babatunde DE, Isaac Fayomi OS, Sadiku ER, Popoola P, Moropeng L, Yahaya A, Mamudu OA (2020) A review on the impact of mining operation: monitoring, assessment and management. Results Eng 8(October):100181. https://doi.org/10.1016/j.rineng.2020.100181
Aparicio JD, Raimondo EE, Saez JM, Costa-Gutierrez SB, Álvarez A, Benimeli CS, Polti MA (2022) The current approach to soil remediation: a review of physicochemical and biological technologies, and the potential of their strategic combination. J Environ Chem Eng 10(2):107141. https://doi.org/10.1016/J.JECE.2022.107141
Arco-Lázaro E, Agudo I, Clemente R, Bernal MP (2016) Arsenic(V) adsorption-desorption in agricultural and mine soils: effects of organic matter addition and phosphate competition. Environ Pollut 216:71–79. https://doi.org/10.1016/j.envpol.2016.05.054
Baker JM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements - a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baragaño D, Forján R, Aguado JMM, Martino MC, García PD, Rubio JM, Rueda JJÁ, Gallego JR (2019) Reuse of dunite miningwaste and subproducts for the stabilization of metal(Oid)s in polluted soils. Minerals 9(8):0–11. https://doi.org/10.3390/min9080481
Baragaño D, Alonso J, Gallego JR, Lobo MC, Gil-Díaz M (2020a) Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 238. https://doi.org/10.1016/j.chemosphere.2019.124624
Baragaño D, Forján R, Fernández B, Ayala J, Afif E, Gallego JR (2020b) Application of biochar, compost and ZVI nanoparticles for the remediation of As, Cu, Pb and Zn polluted soil. Environ Sci Pollut Res 27(27):33681–33691. https://doi.org/10.1007/s11356-020-09586-3
Baragaño D, Boente C, Rodríguez-Valdés E, Fernández-Braña A, Jiménez A, Gallego JR, González-Fernández B (2020c) Arsenic release from pyrite ash waste over an active hydrogeological system and its effects on water quality. Environ Sci Pollut Res 27(10):10672–10684. https://doi.org/10.1007/s11356-019-07120-8
Baragaño D, Forján R, Welte L, Luis J, Gallego JR (2020d) Nanoremediation of As and metals polluted soils by means of graphene oxide nanoparticles. Sci Rep 10(1896). https://doi.org/10.1038/s41598-020-58852-4
Baragaño D, Forján R, Sierra C, Gallego JR (2021a) Nanomaterials for soil remediation: pollutant immobilization and opportunities for hybrid technologies. Sorbents Mater Control Environ Pollut 701–723. https://doi.org/10.1016/B978-0-12-820042-1.00026-2
Baragaño D, Gallego JR, Forján R (2021b) Comparison of the effectiveness of biochar vs. magnesite amendments to immobilize metals and restore a polluted soil. Environ Geochem Health 43(12):5053–5063. https://doi.org/10.1007/s10653-021-00981-4
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158(6):2282–2287. https://doi.org/10.1016/j.envpol.2010.02.003
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202. https://doi.org/10.1016/j.envpol.2013.11.026
Boshoff M, de Jonge M, Scheifler R, Bervoets L (2014) Predicting As, Cd, Cu, Pb and Zn levels in grasses (Agrostis sp. and Poa sp.) and stinging nettle (Urtica dioica) applying soil-plant transfer models. Sci Total Environ 493:862–871. https://doi.org/10.1016/j.scitotenv.2014.06.076
Breure AM, Lijzen JPA, Maring L (2018) Soil and land management in a circular economy. Sci Total Environ 624:1025–1030. https://doi.org/10.1016/j.scitotenv.2017.12.137
Brunetti G, Farrag K, Soler-Rovira P, Ferrara M, Nigro F, Senesi N (2012) The effect of compost and Bacillus licheniformis on the phytoextraction of Cr, Cu, Pb and Zn by three brassicaceae species from contaminated soils in the Apulia region, Southern Italy. Geoderma 170:322–330. https://doi.org/10.1016/j.geoderma.2011.11.029
Clemente R, Hartley W, Riby P, Dickinson NM, Lepp NW (2010) Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ Pollut 158(5):1644–1651. https://doi.org/10.1016/j.envpol.2009.12.006
Du J, Guo Z, Li R, Ali A, Guo D, Lahori AH, Wang P, Liu X, Wang X, Zhang Z (2020) Screening of Chinese mustard (Brassica juncea L.) cultivars for the phytoremediation of Cd and Zn based on the plant physiological mechanisms. Environ Pollut 261. https://doi.org/10.1016/j.envpol.2020.114213
Eighmy TT, Eusden JD (2004) Phosphate stabilization of municipal solid waste combustion residues: geochemical principles. Geol Soc Spec Publ 236:435–473. https://doi.org/10.1144/GSL.SP.2004.236.01.25
Forján R, Rodríguez-Vila A, Cerqueira B, Covelo EF (2017) Comparison of the effects of compost versus compost and biochar on the recovery of a mine soil by improving the nutrient content. J Geochem Explor 183:46–57. https://doi.org/10.1016/j.gexplo.2017.09.013
Forján R, Rodríguez-Vila A, Cerqueira B, Amano M, Asensio Fandiño V, Covelo E, F. (2018a) Assesment of compost and technosol as amendments to increase nutrient contents in a mine soil vegetated with brassica juncea. Span J Soil Sci 8(3):306–321. https://doi.org/10.3232/SJSS.2018.V8.N3.02
Forján R, Rodríguez-Vila A, Cerqueira B, Covelo EF (2018b) Comparison of compost with biochar versus technosol with biochar in the reduction of metal pore water concentrations in a mine soil. J Geochem Explor 192:103–111. https://doi.org/10.1016/j.gexplo.2018.06.007
Forján R, Baragaño Coto D, Arenas Lago D, Gallego Rodríguez JL, Silva Santos E (2021) Effects of different nanoparticles and biochar application on the biological indicators of a polluted mine soil. https://doi.org/10.5194/egusphere-egu21-8247
Gallego JR, Rodríguez-Valdés E, Esquinas N, Fernández-Braña A, Afif E (2016) Insights into a 20-ha multi-contaminated brownfield megasite: an environmental forensics approach. Sci Total Environ 563–564:683–692. https://doi.org/10.1016/j.scitotenv.2015.09.153
García-Manyes S, Jimenez G, Padro A, Rubio R, Rauret G (2002) Arsenic speciation in contaminated soils. Talanta 58:97–109. https://doi.org/10.1016/s0039-9140(02)00259-x
García MA, Chimenos JM, Fernández AI, Miralles L, Segarra M, Espiell F (2004) Low-grade MgO used to stabilize heavy metals in highly contaminated soils. Chemosphere 56(5):481–491. https://doi.org/10.1016/j.chemosphere.2004.04.005
Gong Y, Zhao D, Wang Q (2018) An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: technical progress over the last decade. In: Water research, vol 147. Elsevier Ltd., pp 440–460. https://doi.org/10.1016/j.watres.2018.10.024
Hammond EB, Coulon F, Hallett SH, Thomas R, Hardy D, Kingdon A, Beriro DJ (2021) A critical review of decision support systems for brownfield redevelopment. Sci Total Environ 785:147132. https://doi.org/10.1016/J.SCITOTENV.2021.147132
Hendershot WH, Duquette M (1986) A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci Soc Am J 50(3):605–608. https://doi.org/10.2136/sssaj1986.03615995005000030013x
Hou D, Al-Tabbaa A (2014) Sustainability: a new imperative in contaminated land remediation. Environ Sci Pol 39:25–34. https://doi.org/10.1016/J.ENVSCI.2014.02.003
Houba VJG, Temminghoff EJM, Gaikhorst GA, van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31(9–10):1299–1396. https://doi.org/10.1080/00103620009370514
ICDD (2003) PDF-2 database, release 2003. International Centre for Diffraction Data, Newton Square
Jedynak L, Kowalska J (2011) Stability of arsenic species in hydroponic media and its influence on arsenic uptake and distribution in white mustard (Sinapis alba L.). Microchem J 98(1):163–169. https://doi.org/10.1016/j.microc.2011.01.004
Kisser J, Wirth M, de Gusseme B, van Eekert M, Zeeman G, Schoenborn A, Vinnerås B, Finger DC, Kolbl Repinc S, Bulc TG, Bani A, Pavlova D, Staicu LC, Atasoy M, Cetecioglu Z, Kokko M, Haznedaroglu BZ, Hansen J, Istenič D, … Beesley L (2020) A review of nature-based solutions for resource recovery in cities. Blue-Green Syst 2(1):138–172. https://doi.org/10.2166/bgs.2020.930
Lal R, Bouma J., Brevik E, Dawson L, Field DJ, Glaser B, Hatano R, Hartemink AE, Kosaki T, Lascelles B, Monger C, Muggler C, Ndzana GM, Norra S, Pan X, Paradelo R, Reyes-Sánchez LB, Sandén T, Singh BR, … Zhang J (2021).Soils and sustainable development goals of the United Nations: an International Union of Soil Sciences perspective. Geoderma Regional 25:e00398. https://doi.org/10.1016/J.GEODRS.2021.E00398
Li X, Liu L (2021) Recent advances in nanoscale zero-valent iron/oxidant system as a treatment for contaminated water and soil. J Environ Chem Eng 9(5). https://doi.org/10.1016/j.jece.2021.106276
Ma X, Gurung A, Deng Y (2013) Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci Total Environ 443:844–849. https://doi.org/10.1016/j.scitotenv.2012.11.073
Martínez-Hernández V, Meffe R, Hernández-Martín J, Alonso González A, de Santiago-Martín A, de Bustamante I (2020) Sustainable soil amendments to improve nature-based solutions for wastewater treatment and resource recovery. J Environ Manag 261(January). https://doi.org/10.1016/j.jenvman.2020.110255
Mehlich A (1984) Mehlich 3 soil test extractant : a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15(12):1409–1416
Mudhoo A, Ramasamy DL, Bhatnagar A, Usman M, Sillanpää M (2020) An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol Environ Saf 197:110587. https://doi.org/10.1016/J.ECOENV.2020.110587
Niu A, Lin C (2021) Managing soils of environmental significance: a critical review. J Hazard Mater 417(April):125990. https://doi.org/10.1016/j.jhazmat.2021.125990
O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122. https://doi.org/10.1016/j.advwatres.2012.02.005
Oliveira MLS, Ward CR, Izquierdo M, Sampaio CH, de Brum IAS, Kautzmann RM, Sabedot S, Querol X, Silva LFO (2012) Chemical composition and minerals in pyrite ash of an abandoned sulphuric acid production plant. Sci Total Environ 430:34–47. https://doi.org/10.1016/j.scitotenv.2012.04.046
Palansooriya KN, Shaheen SM, Chen SS, Tsang DCW, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 134. https://doi.org/10.1016/j.envint.2019.105046
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185(2–3):549–574. https://doi.org/10.1016/J.JHAZMAT.2010.09.082
Porta CJ, López-Acevedo Regrerín M, Rodríguez Ochoa R (1986) Técnicas y experimentos en edafología. Collegi Oficial D’ enginyers Agronoms de Catalunya DL
Ray I, Mridha D, Roychowdhury T (2021) Waste derived amendments and their efficacy in mitigation of arsenic contamination in soil and soil–plant systems: a review. Environ Technol Innov 24. https://doi.org/10.1016/j.eti.2021.101976
Rayu S, Karpouzas DG, Brajesh, & Singh, K. (2012) Emerging technologies in bioremediation: constraints and opportunities. Biodegradation 23:917–926. https://doi.org/10.1007/s10532-012-9576-3
Rodríguez-Vila A, Covelo EF, Forján R, Asensio V (2014) Phytoremediating a copper mine soil with Brassica juncea L., compost and biochar. Environ Sci Pollut Res 21:11293–11304. https://doi.org/10.1007/s11356-014-2993-6
Sanchez PA, Couto W, Buol SW (1982) The fertility capability soil classification system: interpretation, applicability and modification. Geoderma 27(4):283–309. https://doi.org/10.1016/0016-7061(82)90019-2
Schjønning P, Elmholt S, Christensen BT (2004) Managing soil quality: challenges in modern agriculture. CABI Publishing, Wallingford, p 344
Song Y, Kirkwood N, Maksimović Č, Zhen X, O’Connor D, Jin Y, Hou D (2019) Nature based solutions for contaminated land remediation and brownfield redevelopment in cities: a review. Sci Total Environ 663:568–579. https://doi.org/10.1016/j.scitotenv.2019.01.347
Stefaniuk M, Oleszczuk P, Ok YS (2016) Review on nano zerovalent iron (nZVI): from synthesis to environmental applications. Chem Eng J 287:618–632. https://doi.org/10.1016/j.cej.2015.11.046
USEPA (1992) US Environmental Protection Agency. Method 1311: toxicity characteristic leaching procedure. https://www.epa.gov/hwsw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure
Vaněk A, Komárek M, Chrastný V, Bečka D, Mihaljevič M, Šebek O, Panušková G, Schusterová Z (2010) Thallium uptake by white mustard (Sinapis alba L.) grown on moderately contaminated soils-Agro-environmental implications. J Hazard Mater 182(1–3):303–308. https://doi.org/10.1016/j.jhazmat.2010.06.030
Vithanage M, Kumarathilaka P, Oze C, Karunatilake S, Seneviratne M, Hseu ZY, Gunarathne V, Dassanayake M, Ok YS, Rinklebe J (2019) Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: a critical review. In Environment International 131. https://doi.org/10.1016/j.envint.2019.104974
Vítková M, Puschenreiter M, Komárek M (2018) Effect of nano zero-valent iron application on as, cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 200:217–226. https://doi.org/10.1016/j.chemosphere.2018.02.118
Wang J, Shi L, Zhai L, Zhang H, Wang S, Zou J, Shen Z, Lian C, Chen Y (2021) Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: a review. Ecotoxicol Environ Saf 207:111261. https://doi.org/10.1016/J.ECOENV.2020.111261
Wcisło E, Bronder J, Bubak A, Rodríguez-Valdés E, Gallego JR (2016) Human health risk assessment in restoring safe and productive use of abandoned contaminated sites. Environ Int 94:436–448. https://doi.org/10.1016/j.envint.2016.05.028
Wickenberg B, McCormick K, Olsson JA (2021) Advancing the implementation of nature-based solutions in cities: a review of frameworks. Environ Sci Pol 125:44–53. https://doi.org/10.1016/J.ENVSCI.2021.08.016
Woolson EA, Axley JH, Kearney PC (1973) The chemistry and Phytotoxicity of arsenic in soils: II. Effects of Time and Phosphorus 1
Wu J, Zhou Q, Huang R, Wu K, Li Z (2021) Contrasting impacts of mobilisation and immobilisation amendments on soil health and heavy metal transfer to food chain. Ecotoxicol Environ Saf 209:111836. https://doi.org/10.1016/J.ECOENV.2020.111836
Zhang Q, Zou D, Zeng X, Li L, Wang A, Liu F, Wang H, Zeng Q, Xiao Z (2021) Effect of the direct use of biomass in agricultural soil on heavy metals __ activation or immobilization? Environ Pollut 272:115989. https://doi.org/10.1016/J.ENVPOL.2020.115989
Zhou W, Liu F, Yi S, Chen Y, Geng X, Zheng C (2019) Simultaneous stabilization of Pb and improvement of soil strength using nZVI. Sci Total Environ 651:877–884. https://doi.org/10.1016/j.scitotenv.2018.09.146
Acknowledgements
Ana Díaz would like to thank the Regional Government of Asturias, from which she obtained a Severo Ochoa grant to develop her PhD thesis (Ref. BP20-192). Diego Baragaño would like to thank the European Union-NextGenerationEU, Ministerio de Universidades, and Plan de Recuperación, Transformación y Resiliencia, through a call of the Universidad de Oviedo, for a Postdoctoral grant (Ref. MU-21-UP2021-030 32892642). We would like to thank the Electron Microscopy Unit and the X-Ray Diffraction Unit of the University of Oviedo for technical support. We are also grateful to Pasek Minerals SAU for supplying the dunite mining waste. The authors thank 3 anonymous reviewers for improving the quality of the paper.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the NANOCAREM project (AEI/Spain, FEDER/EU, MCI-20-PID2019-106939GB-I00).
Author information
Authors and Affiliations
Contributions
A.M. Díaz: Investigation, Formal analysis, Writing. R. Forján: Investigation, Conceptualization, Formal analysis. J.R. Gallego: Project administration, Conceptualization, Funding acquisition. L. Benavente-Hidalgo: Investigation. J.M. Menéndez-Aguado: Resources, Validation. D. Baragaño: Conceptualization, Investigation, Supervision, Writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Michael Komárek.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 41 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Díaz, A.M., Forján, R., Gallego, J.R. et al. Nanoscale zero-valent iron mitigates arsenic mobilization and accumulation in Sinapis alba grown on a metal(loid)-polluted soil treated with a dunite mining waste-compost amendment. Plant Soil 497, 241–255 (2024). https://doi.org/10.1007/s11104-023-05879-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05879-x