Abstract
Background
Biochar-based fertilizer products (BCF) have been reported to increase both crop yield and N-use efficiency. Such positive effects are often assumed to result from the slow-release of N adsorbed on BCF structures. However, a careful review of the literature suggests that actual mechanisms remain uncertain, which hampers the development of efficient BCF products.
Scope
Here, we aim at reviewing BCF mechanisms responsible for enhanced N uptake by plants, and evaluate the potential for further improvement. We review the capacity of biochar structures to adsorb and release N forms, the biochar properties supporting this effect, and the methods that have been proposed to enhance this effect.
Conclusions
Current biochar products show insufficient sorption capacity for the retention of N forms to support the production of slow-release BCFs of high enough N concentration. Substantial slow-release effects appear to require conventional coating technology. Sorption capacity can be improved through activation and additives, but currently not to the extent needed for concentrated BCFs. Positive effects of commercial BCFs containing small amount of biochar appear to result from pyrolysis-derived biostimulants. Our review highlights three prospects for improving N retention: 1) sorption of NH3 gas on specifically activated biochar, 2) synergies between biochar and clay porosities, which might provide economical sorption enhancement, and 3) physical loading of solid N forms within biochar. Beyond proof of concept, quantitative nutrient studies are needed to ascertain that potential future BCFs deliver expected effects on both slow-release and N use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The rationale for making biochar fertilizers
Biochar is the method for increasing soil carbon sequestration that appears to have the most positive interactions with the N cycle, as it tends to reduce N2O emissions and does not require the immobilization of N to build stable C in soils (Guenet et al. 2021). The main rationale for making biochar-based fertilizers (BCF) is that positive interactions between nutrients and biochar in soils can be enhanced through application as a combined product. Retaining nutrients in soils in a plant-available form is considered a key property of biochar amendments (Lehmann 2007). Two decades ago, the highly fertile Terra Preta of the Amazonas were found to contain exceptionally high charcoal contents (Glaser et al. 2001). Charcoal was later proven to be a major component of the nutrient sorption capacity of certain soil types (Mao et al. 2012). These observations suggested that adding biochar to soil could substantially enhance the retention of plant-available nutrients. Biochar-mediated retention and release could in part explain the reported increases in nutrient use efficiency (Chen et al. 2019; Shi et al. 2020, 2022; Zhang et al. 2016; Zheng et al. 2013), decreases in NO3− leaching (Borchard et al. 2019; Chen et al. 2019; Liu et al. 2019a; Zheng et al. 2013) and decreases in N2O emissions (Borchard et al. 2019; He et al. 2017). These positives effects are expected to be greater when nutrients are loaded onto biochar surfaces prior to soil application using BCF technology, and the rationale for this is two-fold. First, in BCF all nutrients are interacting with the biochar surfaces, while nutrients applied to biochar-amended soil have more limited chances for interaction, notably as the distribution of biochar ploughed into soil can be quite heterogeneous (O'Toole et al. 2018). Second, nutrient loading onto fresh biochar can be largely enhanced when biochar is intimately mixed with nutrient sources, heated and/or exposed to varying moisture conditions, such as during composting (Hagemann et al. 2017b; Hagemann et al. 2017c; Joseph et al. 2013; Kammann et al. 2015). Such observations suggest that nutrients could be efficiently bound to biochar prior to soil application. How to do this is a key question, and a central theme of the present paper.
Multiple methods have been used to create BCFs, most of this work being conducted in the last decade. The difficulty in describing and classifying these methods partly comes from the multiple steps and choices to be made when developing a BCFs (Fig. 1). BCFs are composed of 2 to 3 categories of ingredients: 1) biomass feedstock, 2) additives, mostly in the form of clay, minerals and organic substrates, 3) nutrient sources of organic or mineral origin. In addition, activation products can be used, with some of them, such as phosphoric acid, contributing directly to the composition of the BCF (e.g. Carneiro et al. (2018)). These multiple ingredients are combined through a series of successive treatments involving at least two of the following: 1) pyrolysis, including co-pyrolysis with additives, 2) activation of untreated biochar (BCun) with e.g. acids, oxidizers or steam to obtain activated biochars (BCac), 3) mixing of BCun or possibly BCac with additives, to obtain enhanced biochars (BCen) and 4) loading of nutrients onto the BCac or BCen to obtain the BCFs. The mixing and loading phases are sometimes combined as biochar, binding additives and nutrient sources are mixed together.
The simplest method for creating a BCF consists of creating a BCun from a feedstock and loading it through mixing in a nutrient solution. Examples of such methods include soaking BCun in a pure nutrient solution such as urea (Magrini-Bair et al. 2009) or NH4+ in a synthetic form (Cui et al. 2016; Gai et al. 2014; Hale et al. 2013) or the BC can be applied to a more complex organic residue such as manure or digestate (Kizito et al. 2015; Kocatürk-Schumacher et al. 2017b; Kocatürk-Schumacher et al. 2019). In order to directly increase nutrient retention of the BC, activation steps have been used with some success (Huff et al. 2018). Studies have also explored the addition of additives to the BC in order to increase binding and retention of nutrients, such as bentonite clays and organic binders (Joseph et al. 2013). These additives can be added pre- or post-pyrolysis with pre-pyrolysis application having the advantage that the addition can also result in catalysis of the pyrolysis process (Chen et al. 2017; Qian et al. 2014) but with the potential disadvantage that the properties of the additive can be negatively affected (Ismadji et al. 2016). The final mixing and loading of nutrients onto the BCF has often been made through physical blending (Puga et al. 2020; Shi et al. 2020) and co-torrefaction (Joseph et al. 2015; Nielsen et al. 2014; Ye et al. 2016). When manure solutions are used, mixing with minerals followed by heat treatment has been tested in order to ensure a reaction between the BC surface and the additive (Chia et al. 2010; Chia et al. 2014; Lin et al. 2013).
The examples provided above illustrate the diversity of methods used to produce BCFs, and we will further explore their diversity and significance as we link them to mechanisms in this review. The multiple approaches presented above do not represent a chronological development of the technology. For example, producing more efficient BCFs through the use of clays as additive was already reported in 2010 (Joseph et al. 2010). The industrialization of BCF was firstly initiated in 2012 in China and reached commercial scale by 2017, largely based on conventional steam blending of biochar with mineral urea, phosphate and potassium chloride (Pan et al. 2017; Sun et al. 2018).
The development of BCFs so far appears to have been largely empirical, based on trial and error. Many studies have attempted to justify their approach, often a posteriori, by invoking elements of theory such as e.g. sorption and slow release effects as they relate to physico-chemical properties of the composite BCF material. Some elements of theories are well documented, others remain more hypothetical. The magnitude of the reported effects, such as an increase in N use efficiency, has rarely been discussed in light of the theoretical potential of the corresponding BCF-production method. In order to guide product design, we need a better understanding of the mechanisms controlling nutrient loading and release, especially that of N. In the present study, we review and evaluate these crucial elements and their implications in terms of producing BCFs large-scale implementation of biochar in agriculture.
Conditions for BCFs to be actual fertilizers
To define a biochar product as a BCF it must meet the agreed upon definition of a fertilizer. The International Organization for Standardization (ISO) defines fertilizer as “a substance that contains one or more recognized plant nutrient(s), which is used for its plant nutrient content and is designed for use or claimed to have value in promoting plant growth” (ISO 2015). According to this definition, BCFs can be defined as fertilizers if they contain nutrients; however, ISO also indicates that the nutrient content of the fertilizer should meet the law or regulation of each country or region. In the European Union (EU), a new implementing regulation (EU 2019/2164) on organic production and labelling of organic products has been in force since 2020. According to the new regulation, biochar is defined as a “pyrolysis product made from a wide variety of organic materials of plant origin” and is listed in Annex I as an authorized fertilizer. This means that biochar can be used in Europe in organic farming as a fertilizer/soil conditioner. However, it is still not yet authorized as an EU fertilizing product according to EU Regulation 2019/1009 for making fertilizers available on the internal market. This is expected to change in the coming years as the Regulation obliges the European Commission to assess struvite, biochar and ash-based products (STRUBIAS) and biochar to be included as a new component material category in an extended Annex II.
In this regulation, the requirements for several fertilizing product categories are set out. Table 1 summarizes the requirements and contaminant limits for possible fertilizer and soil improver categories for biochar fertilizers according to EU Regulation 2019/1009, if they were listed as authorized fertilizers.
In China, biochar based fertilizers (NY/T 3041–2016, Ministry of Agriculture China) and biochar based organic fertilizer (NY/T 3618–2020, Ministry of Agriculture China) are currently (in 2021) authorized for use in agriculture. As a key ingredient (at least of 5% stable carbon) in such fertilizers, biochar is defined as the solid residue rich in stable organic carbon obtained via oxygen-limited pyrolysis of crop residues at a temperature range of 400 °C – 700 °C. Minimum content of major nutrients of N, P2O5 and K2O is 20% (corresponding to 12.3% of N + P + K) for biochar based fertilizers and 5% for biochar based organic fertilizers, while heavy metals and organics must meet guideline values regulated for fertilizers (Table 2). However, these values are still in debate and need to be updated. Production of biochar and biochar fertilizers are nationally authorized and regulated for development in rural industry in conjunction with poverty reduction in undeveloped areas with plenty of biomass feedstocks, and for use in ecological farming, soil improvement and restoration (GB/Z 39121–2020, China State Agency of Market Supervision and Administration 2020).
Reported effects on plants
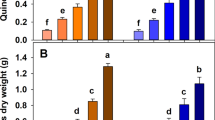
A number of pot and field studies have tested the effects of BCFs on plant growth, but these are comparatively few compared to the number of trials testing pure biochar. In Fig. 2 (and Tables S1), we summarize the main results from 40 BCF observations from 19 studies and report the percentage change in yield of BCFs compared to a fertilized control. Our analysis excludes studies where biochar and fertilizer were added separately to a soil without prior blending. Average plant yield increased by 17% with BCFs as compared to fertilized control, with a standard deviation of 23%. This value is similar to the 15% yield increase reported for biochar and fertilizer applications compared to fertilizer controls in the meta-analysis of Ye et al. (2020), although their review was not limited to blended applications. The high variability of the response reflects the diversity of 1) biochars used in the BCF, 2) production methods and 3) experimental conditions in terms of plant species and soil types. The studies used in Fig. 2 span a range of formulation methods and ingredients including: 1) torrefaction of biochar with clay, minerals, inorganic and organic fertilizers; 2) mixing and incubation of biochar with liquid manures, digestate or urine; 3) physical blending of biochar with inorganic fertilizers, including heat treatment of the mixture for improved bonding and coating methods. A full list of data from the studies are included in supplementary information (Table S1) and a summary of chemical properties of BCFs averaged across multiple studies are given in Table 3. Here we see that the C content of BCFs is approximately half that of pure biochar, the latter ranging from 60 to 90%. In these studies, the BCFs were enriched in N, P, K, and displayed a high pH and a low surface area, the latter probably due to coating or filling of pores with clay and fertilizer (Table 3).
Mean ± SD Crop yield change with BCF compared to a fertilized control, grouped by BCF N source and whether clay was an ingredient (outliers excluded). Based on studies: Zheng et al. (2017), Ye et al. (2020), Yao et al. (2015), Wen et al. (2017), Shi et al. (2020), Qian et al. (2014), Puga et al. (2020), Nielsen et al. (2014), Magrini-Bair et al. (2009), Liao et al. (2020), Kocatürk-Schumacher et al. (2019), Joseph et al. (2015), González et al. (2015), Farrar et al. (2019), El Sharkawi et al. (2018), Chew et al. (2020), Blackwell et al. (2015), Schmidt et al. (2017) [NPK treatment], Liao et al. (2020). (outliers excluded – Schmidt et al. (2015) [306%+], Schmidt et al. (2017) [123%+]
Mechanisms responsible for stimulation of plant yield by BCFs are still under investigation, but in summary the majority of studies stated either a slow release effect or increased N use efficiency (NUE), which they partly attributed to: 1) pH-change effects on microbial communities (Nielsen et al. 2014), 2) increased root growth and N uptake (Liu et al. 2020b; Shi et al. 2020; Xiang et al. 2017), 3) increased mycorrhizal root colonization (Blackwell et al. 2015), 4) increased physical retention of dissolved nutrients and reduced leaching (El Sharkawi et al. 2018; Schmidt et al. 2017; Schmidt et al. 2015; Shi et al. 2020; Wen et al. 2017), 5) increased nitrification (Liao et al. 2020), 6) improved redox conditions and changes in abundance of growth promoting micro-organisms (Chew et al. 2020), 7) increased P and K availability (Farrar et al. 2019), and 8) slower diffusion of NH4+ and NO3− to soil solution (Liao et al. 2020).
A number of studies reported increases in NUE but did not suggest responsible mechanisms (Joseph et al. 2015; Qian et al. 2014; Yao et al. 2015). On average, nutrient use efficiency increased by 34% ± 27 (n = 5). While most of the experiments are controlled with respect to N amounts, it is difficult to simultaneously control for P and K as well, especially when using digestate and other P and K-rich organic fertilizers. Urea was used in 88% of formulations where biochar was combined with mineral N fertilizer. In general, clay was included as an ingredient in 71% of the BCF formulations reported, suggesting that interactions between biochar and clay may play a key role in potential yield benefits, as will be later discussed in this review.
The theory of N-efficient BCFs
Nitrogen use efficiency is defined as the ratio between N outputs in harvested products over total field N inputs (Zhang et al. 2015). Improvements in fertilizer technology are key to increase NUE (Fageria and Baligar 2005), and it has been reported that BCFs increase NUE compared to soluble mineral fertilizers (Chen et al. 2019; Shi et al. 2020; Zeng et al. 2013). This is an important incentive for the development of BCF products.
Effects of BCF on N-use efficiency, as compared to that of mineral fertilizer, are summarized in Fig. 3. The difference between N inputs and outputs, also referred to as the N surplus, is either stored in the soil or lost from the soil system. When stored in the soil, the N surplus can contribute to SOM build-up (Soussana et al. 2017; van Groenigen et al. 2017). However, in the absence of a SOM management strategy, loss can predominate (Zhang et al. 2015). The efficiency of mineral N fertilizers is limited both by gaseous losses of N2, N2O and NH3 and by leaching losses, predominantly as NO3− (Xiang et al. 2020). Biochar-induced reductions in N2O losses can result from a higher proportion of NO3− being converted to N2 as compared to N2O (Harter et al. 2013; Weldon et al. 2019), meaning that reductions in N2O emission do not necessarily indicate a reduction in gaseous N loss. By contrast, runoff and leaching losses of N have been hypothesized early on to be drastically reduced by biochar products (Magrini-Bair et al. 2009). Enhancement of root growth by BCF application is an additional factor that might contribute to improved N capture and retention in soils (Yan et al. 2020).
Increase in NUE with BCF has largely been attributed to a putative slow-release effect that biochar matrices have on N fertilizers (Cai et al. 2016; Dong et al. 2019; Ibrahim et al. 2020; Manikandan and Subramanian 2013). However, when mineral N was loaded on wood and sludge biochar surfaces, Keskinen et al. (2021) reported no observable slow release effect. This finding is not surprising given that sorption and desorption of N on biochar surfaces is a rapid process often occurring on timescales of less than a day (Kizito et al. 2015; Wang et al. 2020). A fertilizer is considered as slow release if it releases less than 15% and 80% of its N after 24 h and 28 d, respectively (Jia et al. 2020). A slow-release effect can be obtained either with fertilizer products of limited solubility (ISO. 2015) or through coating with a protective layer that requires hydrolysis and/or biodegradation before the fertilizer is released (ISO 2015; Xiang et al. 2020). Biochar has been tested as an ingredient in coating material for urea, showing promise for improving the slow-release effect, especially when using high-temperature biochars with high surface area (Jia et al. 2020). However, the use of a conventional protective coating on top of the biochar layer appears crucial for obtaining this slow release effect (Jia et al. 2020; Khajavi-Shojaei et al. 2020). Examples of such coatings include starch and polyvinyl alcohol, which have been successfully used to produce slow-release BCFs (Dong et al. 2019; Gwenzi et al. 2018; Liao et al. 2020; Liu et al. 2019b; Sim et al. 2021).
Biochar properties supporting fertilizer-based functions
Biochar is often reported to be an exceptional product for retaining nutrients in soils due to its high cation exchange capacity (CEC) and high porosity (Liang et al. 2006; Lychuk et al. 2015; Wong et al. 2019). Assuming that this widely held view is true, a logical hypothesis is that biochar should also make an outstanding support for delivering nutrients to plants in the form of compound fertilizers. Exploring this hypothesis requires first a better understanding of the nature of the CEC and porosity of biochars.
Biochar surfaces can exchange both anions and cations, with the CEC increasing with pH while the anion exchange capacity (AEC) displays the opposite response (Lawrinenko and Laird 2015). The CEC of biochar results from oxygenated functional groups on biochar surfaces such as carbonyl, carboxyl and hydroxyl groups (Suliman et al. 2016). Untreated biochars are high pH products, mostly in the 8–10 pH range (Budai et al. 2014). At desirable pH values for agricultural soils, the CEC largely predominates over the AEC (Silber et al. 2010). Although some of the AEC appears pH independent, its contribution to total ion exchanges remains low (Lawrinenko and Laird 2015; Silber et al. 2010). For this reason it may be expected that biochar capacity for adsorbing cationic nutrients will be of greater significance for the development of a nutrient rich BCF, and therefore we specifically address the CEC of biochar in the following section. Higher CEC values are caused by an abundance of functional groups, while the process of pyrolysis under increasing treatment temperature is largely one of aromatization at the cost of functional groups. This is why the highest CEC of biochar products is often obtained at a fairly low treatment temperature, ~400 °C for slow pyrolysis conditions (Amin 2020; Budai et al. 2014; Kameyama et al. 2017; Singh et al. 2020; Wu et al. 2012). It also explains why hydrothermal carbonization and low-temperature carbonization products, obtained at about 250 °C, often exhibit a high CEC (Amin 2020; Budai et al. 2014; Mukherjee et al. 2011). However, the stability in soils of ~250 °C pyro- and hydrochars is at least an order of magnitude lower than that of biochar produced over 370 °C (Budai et al. 2016), making the former products unusable for carbon-sequestration co-benefits.
Ageing in soils increases the CEC of biochars (Lehmann 2007). This effect is attributable to the increased oxidation of surface groups (Liang et al. 2006) and possibly to the binding onto biochar of high-CEC molecular structures having properties similar to those of humic acids (Liang et al. 2006; Wiedner et al. 2015). Very high CEC of biochar has been inferred from the properties of aged charcoal in soils (Liang et al. 2006), while the CEC of fresh biochar is usually rather low (Budai et al. 2014). Studies looking at the short-term increase in the CEC of soil following high-dose biochar application report either no increase (Basso et al. 2013) or only modest increases (Laird et al. 2010), with the largest relative increases reported for soils very low in clay and soil organic carbon and hence low CEC (Cornelissen et al. 2013). It is therefore crucial not to confuse the CEC of fresh biochar with that of its aged forms in soils, especially as we know little about the dynamics and timeframe of the oxidation and loading processes in soils (Lehmann 2007) or organic environments (Hagemann et al. 2017b; Prost et al. 2013). However, observations of biochar in soils have interesting implications for biochar-fertilizer applications, specifically the possibility to increase the CEC of biochar through artificial oxidation and/or coating with high CEC organics, as discussed later.
Reported CEC of biochar products is quite variable (Table S2, Fig. 4.) due to factors affecting the surface properties of biochar, such as feedstock type and pyrolysis temperature (Jellali et al. 2022; Mukherjee et al. 2011), but also due to variability and errors in the analytical methods (Munera-Echeverri et al. 2018). Notwithstanding possible high-value artefacts, the maximum CEC value of biochar products seems to be at around 80 cmolc kg−1 (Fig. 4; Zwart 2020). However, most reported values for biochar CEC are in the range of 1 to 35 cmolc kg−1, with wood-based biochar at the lower end of the range (Fig. 4). Accordingly, Lychuk et al. (2015) consider that a biochar with a modest 18 cmolc kg−1 is a high CEC product. By contrast, some clay minerals and zeolites display a much higher CEC, i.e. up to 150 cmolc kg−1 for vermiculite clay (Christidis 2013) and up to 210 cmolc kg−1 for zeolites (Koon and Kaufman 1975). For this reason, clay minerals and zeolites are known to be effective sorbents for inorganic cations including NH4+ from various solutions, due to their high CEC and high specific surface area (Abollino et al. 2008; Christidis 2013). The limited CEC of biochar consequently appears to restrict its ability to retain NH4+ as compared to some other sorbents (Kocatürk-Schumacher et al. 2017a). This is confirmed by several studies reporting higher removal of NH4+ from solutions and a higher concentration of nutrients in the sorbent with zeolite as compared to biochar (Carey et al. 2015; Hina et al. 2015; Kocatürk-Schumacher et al. 2017a). Based on the CEC values mentioned in this section, one kg of biochar, vermiculite and zeolite loaded with NH4+ could theoretically deliver approximately 3, 20 and 30 g N for plant growth, respectively. This might also explain why most studies reporting positive yield effects with BCF have used clay minerals as additives (this review, see Fig. 2).
Range of cation exchange capacity (cmolc kg−1) of various minerals (Koon and Kaufman 1975; Christidis 2013; Zwart 2020) and of several biochars produced from various feedstocks and at various pyrolysis temperatures, based on the studies; Budai et al. (2014), Cui et al. (2016), Gai et al. (2014), Hale et al. (2011), Huff et al. (2018), Jassal et al. (2015), Kocaturk-Schumacher et al. (2019), Li et al. (2018), Mia et al. (2017), Munera-Echeverri et al. (2018), Singh et al. (2020), Zeng et al. (2013), Zheng et al. (2017)
The magnitude of N sorption on biochar surfaces
We undertook a detailed search of the literature to identify studies that quantified the sorption of NH4+ and NO3− on biochar surfaces based on batch studies (Table S3 and S4). Batch studies are valuable because they represent a highly repeatable and controlled measure for the sorption properties of a material. Our focus was to examine the maximum sorption potential of unmodified biochar across a range of feedstock and pyrolysis temperatures. We converted all values to mg NO3-N or NH4-N per g biochar.
A recent meta-analysis of sorption studies conducted on untreated biochar reported an average maximum sorption potential of 11.2 and 1.78 mg N g−1 biochar for NH4+ and NO3− respectively, based on the modelled estimate provided by the Langmuir coefficient Qmax (Zhang et al. 2020b). Here, we extend this data set with values reported from single concentration batch studies. Our synthesis of NH4+ and NO3− maximum sorption potentials reported in the literature are highly skewed (Table 4), suggesting that the median value is a more reliable estimate than the mean. Across studies, the median sorption potential is modest, i.e. 5.6 mg g−1 for NH4-N and 0.18 mg g−1 for NO3-N. This median sorption value for NH4+ appears consistent with average biochar CEC reported in the previous section, providing further evidence for the fairly low capacity of biochar products to sorb NH4+. Our review of the published data also suggests that source article has a greater impact on the maximum nutrient sorption than either BC highest treatment temperature (HTT) or choice of feedstock (Fig. 5. a,b,c,d). Studies that measure high values report results of similar magnitude across both BC HTT and feedstock gradients, such as in Gao et al. (2015) and Takaya et al. (2016). This suggests that specificity of experimental set-up and methodology might be the primary explanation for the highest reported N sorption values on biochar surfaces. Repeating such studies would be highly valuable. As an example of methodology effect, Wang et al. (2015) showed that NH3 volatilisation, due to alkalisation by high pH biochar, could result in overestimates of the true sorption potential of char for NH4+ by as much as 39%.
a, b: NH4+ maximum sorption. c, d: NO3− maximum sorption. a, c: Feedstock type. b, d: BC HTT. Point colours group by study. Feed type defined after Cayuela et al. (2014)
The apparent strong effect of study methodology on the maximum sorption potential of NH4+ and NO3− makes it difficult to define an accurate value or predict which feedstock or BC HTT combination will yield the best results. A dedicated study conducting standardized reanalysis and modelling of published data would be necessary to sort out methodological effects from biochar production conditions. Notwithstanding our current limited understanding of variability, the question of a realistic and achievable higher-end value for the sorption of NH4+ on biochar surfaces remains. From our current knowledge, we suggest that the upper quartile of the distribution is a reasonable estimate, i.e. 3 and 20 mg N g−1 for NO3− and NH4+ respectively (Table 4). Based on these estimates for high sorption capacity biochar products, an annual fertilisation rate of 120 kg N ha−1 would require application of 6 t ha−1 and 40 t ha−1 of biochar charged with either NH4+ or NO3− respectively. However, how to reach these higher values is not yet understood.
In addition to NH4+ in solution, sorption of N in the form of NH3 has attracted attention in recent years. Although NH3 sorption can happen in high pH solutions (Park et al. 2019), it is mostly sorption from gas streams that is of interest. While untreated biochar displays only limited sorption for NH3 gas, i.e. in the range of m 0.15 to 5.09 mg·N g-1 (Ro et al. 2015), most promising results have been obtained in experiments using biochar treated with oxidants or acids (Hestrin et al. 2019; Krounbi et al. 2020; Ro et al. 2015). Under pure NH3 atmosphere, oxidized biochar has been reported to fix between 40.6 and 90.3 mg N g−1 (Hestrin et al. 2019; Krounbi et al. 2020). Ro et al. (2015) were able to fix up to 52 mg NH3-N per g of biochar activated with phosphoric acid in a continuous-flow experiment with 103 ppm NH3 gas. Remarkably, in the latter study, the activated biochar proved to be a perfect scrubber before starting to saturate.
The importance of surface area and porosity
The high porosity and high surface area of biochars is often quoted as a key element supporting a high exchange capacity for nutrients (e.g. Lychuk et al. 2015). However, the porosity of biochar is difficult to characterize and study because pore diameters span five orders of magnitude (Brewer et al. 2014). Biochar has a macroporosity inherited from the structure of the plant material it was made from, typically from a few microns to a few tens of a micron. This macroporosity is crucial for increasing the retention of plant-available water in soils (Cornelissen et al. 2013; Obia et al. 2016), which is a key beneficial effect of biochar in soils (Razzaghi et al. 2020). This macroporosity, which is inherited from the plant structure, does not appear to be influenced by pyrolysis temperature (Hyvaluoma et al. 2018). Large amounts of solution can remain entrapped in both the macro and the mesopores of biochars. When considering making BCF, this can be a highly concentrated fertilizer solution, which may dry out as concentrated fertilizer deposits in biochar pores. Since the macro-, meso- and nanopores of biochars constitute a labyrinthine structure, asymmetric, hydrated molecules such as NO3− may need time to diffuse from an inner site within a biochar particle to the outside (e.g. alongside a gradient built up by plant roots), depending on moisture and temperature conditions around and in the particle (Conte et al. 2014). The different pore sizes in biochar particles may also separate nutrients in some macropores, mesopores and nanopores from microbial access, since microbes (mostly above 1 μm diameter) will not be able to access such small pores, while plant roots in the vicinity of biochar particles might be able to build up ion-concentration gradients to empty such pores. Such a ‘mole sieve’ effect of biochar, excluding denitrifiers, is one of many explanations for the reduction of N2O emissions observed when biochar is applied to soil (Borchard et al. 2019; Kammann et al. 2012; Kammann et al. 2015). High macroporosity might also be key for adding specific products in concentrated BCFs, such as those based on infiltration of molten urea in biochar particles (Wang et al. 2021; Xiang et al. 2020).
Nanoscale porosity governs sorption dynamics and is borne by nanometric to sub-nanometric pores in the polyaromatic structure of biochars (Brewer et al. 2014). This porosity is generally estimated through gas sorption methods, with CO2 for pores less than a nanometer and N2 for larger ones (Brewer et al. 2014). There is increasing evidence that, rather than surface area, it is pH-dependent ion exchange mechanisms that are largely responsible for the sorption of major N sources such as NH4+ (Fidel et al. 2018), NO3− (Heaney et al. 2020) and urea (Singh et al. 2020). Therefore, the significance of biochar surface area for the uptake of nutrients appears uncertain.
Although some early studies suggested that the CEC of biochar and its surface area would both increase with pyrolysis temperature (Lehmann 2007), we now know that it is not the case because of the aromatization trade-off between loss of functional groups and gain in surface area (Budai et al. 2014; Singh et al. 2020). It is likely that for NH4+, CEC is the dominant factor. For urea sorption, Singh et al. (2020) report that maximum sorption is obtained with BC HTT at 450 °C, and they further argue that this temperature corresponds to an optimum between CEC and surface area for sorption. Slow pyrolysis biochars produced at around 400 °C appear therefore to have the highest potential for sorbing cations such as NH4+.
Improvement through acid and base treatments and oxidations
As previously discussed, studies that attempted to sorb nutrients such as NH4+ and NO3− on untreated biochar structures generally reported fairly low values (Table 4; Fig. 5.). Such low values for N retention on unmodified biochar do not meet expectations for a BCF product. For this reason, efforts have been put into modifying biochar in order to optimise nutrient retention. Several methodologies, including treatments with steam as physical activation, and chemical activation have been proposed to modify both the physical and chemical properties of biochars (Sizmur et al. 2017). Chemical activation with acids, bases and salts have been undertaken both as the post treatment of biochars and as pretreatments of the biomass prior to pyrolysis (Blackwell et al. 2015).
The activation can result in the increased ability of biochar to adsorb cations, anions and organic molecules. Huff et al. (2018) reported that the CEC of pine wood biomass produced at 400 °C doubled from about 15 to 30 cmolc kg−1 through ozone treatment. Acid treatment of biochars enriched with clay and Fe may increase surface area, CEC and silica content through leaching out impurities and changes to the surface charge (Lin et al. 2013) but further research is needed to verify such findings.
A considerable amount of research has been published over the last 15 years regarding changes in biochar properties as a result of post-pyrolysis activation. Laboratory trials have been carried out using strong acids such as HNO3 and H2SO4 (Liu et al. 2012; Qian and Chen 2014) and weak organic acids such as citric and malic acids (Heaney et al. 2020; Lonappan et al. 2020). Most base treatments have been carried out using NaOH and KOH (Liu et al. 2012; Petrovic et al. 2016) and salt treatments have relied on chlorides (Zhang et al. 2020a).
Activation of biochar using acid and alkali solutions might be expensive at large scale and requires careful disposal of the activation media (Sizmur et al. 2017). Oxidation with hydrogen peroxide (Mia et al. 2017; Wang et al. 2015; Xue et al. 2012) and ozonation (Huff et al. 2018) have also been proposed as alternative post-treatments to increase sorption capacity.
Chemical activation of biochar leads to higher porosity and CEC, more oxygen functional groups as well as a higher concentration of water extractable organic compounds (Lawrinenko et al. 2016; Lin et al. 2012). Activation increased NH4+ sorption by 10 mg N g−1 using H2SO4 on bamboo biochar (Asada et al. 2006), by 12.8 mg N g−1 using both HNO3 and NaOH on corncob biochar (Nguyen et al. 2019), and by 5 mg N g−1 using H2O2 on corncob biochar (Wang et al. 2016). The latter value was four times that of the untreated biochar. Even though these literature findings suggest an improved NH4+ retention on biochar as a result of post- treatment with activation and oxidation treatment, the amount of NH4+ retention still appears limited.
In summary, surface treatment of biochar with acids, bases and oxidants has the potential to modify the surface properties of biochar, which can improve nutrient retention. However, there is little research examining the consequences of these treatments on other desirable properties of the BC. Washing of char, especially with strong acids, bases and oxidants has the potential to leach potentially valuable components off the char. This can include the mineral ash, which is largely responsible for the observed liming effect of biochar (Fidel et al. 2017), and dissolved organic carbon (DOC), which could be responsible for mediating sorption of nutrients (Mia et al. 2017) or otherwise stimulating plant growth and development (Liu et al. 2020a). Additionally, severe chemical oxidation may have detrimental effects on the biochar porous structure and could alter its stability in soil (Duan et al. 2019; Li et al. 2019). This means that prior to application of these techniques there needs to be careful consideration of the potential trade-offs associated with these treatments in light of the desirable properties of the BCF.
Improvement through mineral and clay addition
The properties of biochars can be improved through the addition of minerals either as a pretreatment of the biomass or a post treatment of the biochar (Chia et al. 2014; Farrar et al. 2019). Rawal et al. (2016) found that the addition of clay and iron sulphate to bamboo biomass prior to pyrolysis increased both the concentrations of condensed aromatic, acidic, and phenolic carbon species. Some of these effects might be beneficial to the N cycle, however they also report that co-pyrolysis with clay and iron increased N volatilization losses from the BCF. Viglašová et al. (2018) found that pretreating bamboo with montmorillonite and pyrolysing at HTT of 460 °C doubled the maximum adsorption of NO3−. Similarly, higher NO3− removal efficiency was obtained by incorporating Mg/Fe double hydroxides into wheat straw biochar (Xue et al. 2016) and Fe/Ni nanoparticles on sugar cane bagasse biochar (Li et al. 2017). Other studies have shown improvements in specific properties by adding zero valent iron, basalt, or amorphous silica.
Clay-biochar composites have been designed and tested for their ability to sorb NH4+ (Chen et al. 2017; Huang et al. 2020; Ismadji et al. 2016). Chen et al. (2017) studying a bamboo biochar-montmorillonite composite reported an increase in the maximum sorption capacity for NH4+ of 412% from 2.44 mg g−1 to 12.5 mg g−1. In a study by Ismadji et al. (2016) a cassava peal biochar combined with bentonite resulted in a 149% increase in NH4+ sorption, from 9.49 mg g−1 to 23.67 mg g−1. Chen et al. (2017) concluded that the addition of clay prior to pyrolysis acted as an acid catalyst, which fostered reduction processes; however they do not report the sorption capacity of the clay alone. Ismadji et al. (2016) identified a composite effect where the combination of BC and bentonite resulted in a higher sorption capacity than the BC and bentonite alone, apparently due to the fact that the bentonite displayed a finer porosity structure (mesoporous) when combined with the biochar. Yao et al. (2014) reported that clay addition increased the sorption capacity of biochar for methylene blue. They also observed that the effect was more pronounced with bagasse biochar than with bamboo or hickory biochars, and with montmorillonite than kaolinite clay. To increase binding of clay to biochar particles, Huang et al. (2020) used Na2SiO3. Liao et al. (2020) suggest that bentonite fixed inside biochar pores swells in contact with water and thereby slows down the diffusion of urea from the BCF to the soil solution. The clay treatment does not appear to substantially increase the surface area of the biochar, but it increases its capacity for ion exchange (Yao et al. 2014). When combining chicken manure with biochar through torrefaction, Lin et al. (2013) report that addition of clay helped conserve N in the resulting BCF. The use of bentonite in BCF increased yield while reducing NO3− content of pepper plants (Yao et al. 2015). Shi et al. (2022) pelleted maize straw biochar with bentonite and sepiolite amended with carboxymethyl-cellulose and blended with commercial urea for wheat production. As shown in their field test, the NUE reached 42% compared to 33% for conventional urea without biochar and clay.
Improvement with organic coating
The interaction of biochar with organic matter in soils has long been postulated to have a significant role in the development of biochar effects on plant yield and nutrient retention (Hagemann et al. 2017b; Kammann et al. 2015; Lehmann et al. 2002; Liang et al. 2006; Sarkhot et al. 2013). The organic coating of biochar is often cited alongside other natural aging processes to explain the greater nutrient retention potential of aged biochar (Fischer and Glaser 2012; Hagemann et al. 2017a; Liang et al. 2006). However, so far research has focused primarily on the oxidation of biochar to explain the effects of aging on biochar properties (Liang et al. 2013; Wang et al. 2015). Very little mechanistic work has been undertaken to understand the significance of organic coatings for mediating these effects and how they might be exploited in the creation of a commercially viable BCF. Here we consider what is known about the effects of biochar and organic matter interactions on the uptake and retention of NH4+ and NO3−.
Ammonium is a prime candidate for sorption to biochar due to both the alkalinity and the generally larger CEC than AEC of biochar. However, as we have already discussed, the CEC of many unmodified biochars is relatively low in comparison with that of soil organic matter. Several studies report that the sorption capacity of biochars for NH4+ increases when organic molecules bind to biochar surfaces (Lehmann et al. 2002; Sarkhot et al. 2013; Conte and Laudicina 2017). Consistent with this effect, co-composting was reported to increase the CEC of a hardwood biochar from about 3 to about 18 cmolc kg−1 Khan et al. (2016). Prost et al. (2013) showed that the surfaces of co-composted biochar acquired dissolved organic molecules and nutrients from the compost. Although these organic molecules could also compete for and occlude the same polar functional groups responsible for the biochar CEC, it is thought that the net result is an increase in the sorption capacity of the biochar for plant nutrients (Conte and Laudicina 2017).
Nitrate sorption by biochar also appears enhanced by organic coatings. When untreated, biochar generally displays low NO3− sorption capacity, which is consistent with their low AEC values (Hale et al. 2013; Hollister et al. 2013). However, soils amended with large quantities of biochar (1.5 to 6% w/w) have been shown to retain NO3− as compared to no-biochar controls (Chen et al. 2019; Haider et al. 2015). Kammann et al. (2015) observed that a co-composted biochar had captured both anionic and cationic nutrients, with the largest fraction as NO3− (up to 5.3 g N kg−1). Hagemann et al. (2017c) confirmed this NO3− “capture” phenomenon with different woody and sewage-sludge biochars, with a ~ 700 °C wood biochar having the largest effect. Hagemann et al. (2017b) were able to demonstrate that an organic coating rich in N and N-containing functional groups forms on biochar particles during co-composting. It was hypothesized that NO3− anions may be trapped in DOC- or clay-clogged biochar nanopores, retarding their release to the surrounding soil (Joseph et al. 2018).
The results reported here may serve as a starting point to develop biochar products with enhanced environmental N effects, such as reduced NO3− leaching and N2O emissions. However, the positive effects of organic coatings on NO3− retention reported here have generally been obtained in studies using large biochar applications (>1 w/w %). Although some studies report large relative enhancements of NO3− retention, the values obtained remain low in absolute terms (below 5 mg NO3-N per g biochar). The same applies to increased NH4+ retention, the increase is comparatively large but remains limited in absolute values. Biochar coated with organics are currently in the low range of biochar/N ratio when considering BCFs (Fig. 6.), and substantial improvement would be needed for actual fertilizer applications. The technology of organic coatings might be better suited for biochar amendments for environmental applications than for BCFs, but at this point we know too little to exclude that significant breakthroughs could be made towards BCF applications, and more research is clearly needed on this topic.
Biochar-mediated uptake of nutrients
As we have previously discussed, BCF can increase the NUE of plants (Chen et al. 2019; Shi et al. 2020; Zeng et al. 2013). However, sorption-like processes are not the only possible mechanisms leading to an enhancement of NUE with BCF. In this section we investigate how biochar applied in fairly low amounts like in current BCF may trigger plant responses that also result in a more efficient uptake of nutrients by crops.
Nutrient availability in soil is affected by microbial communities (Brussaard et al. 2007), which can in turn be affected by the presence of biochar (Budai et al. 2016). This suggests that the interaction between BCF and soil microbes might affect the availability of native soil nutrients as well as the uptake of the nutrient applied with the BCF. Ye et al. (2016) observed that a biochar-treated compost increased total soil NO3− more than compost alone, and attributed this effect to the stimulation of soil nitrifier populations. In the same study, however, they also noted that biochar/compost addition, which had a relatively high pH, reduced the amount of plant available P. Similar studies also revealed that BCFs applied at low rates (100–1000 kg ha−1) can increase root colonization by arbuscular mycorrhizal fungi (Blackwell et al. 2015), increase the abundance of N fixing bacteria and increase the bioavailability of P, K and N (Nielsen et al. 2014). Chew et al. (2020) measured an increase in rice biomass using a BCF containing urea, Fe2O3 and apatite. They attributed this effect to increased soil pH, Eh and a shift in microbial community composition. They also discovered that the BCF treatment induced changes to the root membrane potential of the rice, which they hypothesized resulted in greater potential nutrient uptake by the plant.
In addition to the proposed biochar effect on root uptake, studies have also measured an increase in root development following biochar addition (Xiang et al. 2017). This is especially true in infertile soils or in rain fed regions, where more abundant root hairs can increase the ability of plants to access nutrients in soil (Liu et al. 2020b). There is some discussion regarding the mechanism by which biochar stimulates root development. The most basic explanation is that the biochar addition alters soil physical properties allowing better root development without the adverse effects of physical barriers such as soil compaction (Amendola et al. 2017; Omondi et al. 2016). However, recent studies have suggested that the soluble components of biochar may play a more important role in stimulating plant root growth following biochar addition (Kolton et al. 2017; Lou et al. 2016). Liu et al. (2020a) investigated the effect on maize growth of different biochar components such as water-soluble biochar extract, mineral nutrients in ash and washed biochar. They found that the addition of water-soluble biochar extract promoted maize growth accompanied with greater root size, longer root hairs and more root tips. It was hypothesized that this effect was due to the presence in the biochar of hormone-like substances, which promoted root development. However, further studies are needed to verify this effect using a range of soil conditions, different biochar feedstocks and pyrolysis conditions.
Implications for biochar-based fertilizer design
Application rates, ease of use in the field and effects on yield will be key factors determining the adoption of BCFs by farmers. In this review, we hypothesize that farmers will be using BCFs to cover part or all of their annual fertilizations needs, meaning that we are not considering one-time large amount applications. When based on mineral fertilizers, BCF products will need pelletization or granulation for ease of application. A balanced mineral NPK fertilizer contains typically 15% N, thereby requiring application rates of about 1 ton ha−1 for delivering 150 kg N ha−1. For a concentrated fertilizer product, for example in pellet or granule form, a 50% biochar mix is likely an upper limit, i.e. one ton of biochar per ha. The upper mixing ratio between N and biochar would therefore approximate 1:7. Based on N sorption properties in solution, our review suggests this ratio could reach approximately 1:150 for untreated biochar, 1:100 for activated biochar, 1:50 for current enhanced biochar with additives (Fig. 6). These values are too high for practical purposes, and the question remains if future biochar-based sorbents can be developed to reach a N:biochar ratio as high as 1:5. It is notable that current concentrated BCFs based on physical blends, i.e. non-sorption, contain as little as 20% biochar in the fertilizer mix (Shi et al. 2020), which translates into a ~ 1:1 N:biochar ratio (Fig. 6). For such products, the main effect is not thought to come from sorption processes, but would rather result from pyrolysis-derived biostimulants promoting root growth or beneficial micro-organisms, as discussed in previous sections.
When using concentrated N sources, our review also indicates that sorption is not the only way to load N onto biochar for BCF purposes. Physical protection of low solubility N forms within biochar structures could provide high-N content and slow release. The case of infiltration of molten urea into biochar structures is an interesting example of such applications, where nearly complete and slow release of the urea N was obtained (Xiang et al. 2020; Wang et al. 2021). Such application deserves further research, notably in terms of the pore distribution of the biochar needed to maximize this effect, as well as the chemical reactions taking place when using molten urea. Dissolution of precipitated or solidified products within biochar structures might be slowed down when using larger biochar particles, following mechanisms already proposed for desorption processes (Kang et al. 2018). This suggests that suitable combinations of biochar porosity and particle size might be a promising optimization for non-coated high-N BCFs.
Nitrogen contained in organic waste streams such as manures could also be a prime target for making BCFs, presenting a win-win solution of reducing pollution risks and increasing yields. Though manure composition varies, most of its N is generally present in the form of NH4+, which is mineral and cationic (Montégut et al. 2016; Portejoie et al. 2004). Therefore, trying to fix manure N on biochar surfaces presents challenges similar to those encountered with mineral N sources. Transferring N from liquid manures into BCFs will require biochar with super sorbent properties in a liquid phase, which remains a future prospect. However, our review points to some interesting prospects regarding effects of clay addition and NH3 gas sorption on oxidized biochar surfaces.
Interaction with clays deserves greater scrutiny. We have seen that most studies reporting increased NUE effects used clay-enhanced BCFs. Certain clay materials display CEC values substantially higher than those of biochar (Fig. 4) and the study of Ismadji et al. (2016) also suggests that biochar has positive synergistic effects on clay porosity for sorbing nutrients. Effects beyond simple additionality is precisely what we look for in making BCFs.
Adsorption of NH3 gas on biochar surfaces appears to reach high values when biochar is activated with acids or oxidants, as detailed in a previous section. Values of 40 to 90 mg NH3-N per g biochar (Hestrin et al. 2019; Krounbi et al. 2020; Ro et al. 2015) are well with the future sorbent category presented at Fig. 6. In addition, NH3 adsorbed on biochar surfaces appears to be plant available (Krounbi et al. 2021; Taghizadeh-Toosi et al. 2012). High affinity of activated biochar for NH3 gas is especially significant as NH3 volatilization from animal manure is a major source of pollution from agriculture and represents huge annual N losses of about 2 and 10 million tons N per year for the European Union and China respectively (Paulot et al. 2014; Xu et al. 2017). Using activated biochar to capture NH3 gas appears therefore as a venue that should be further explored where biochar filter materials are turned into BCFs. However, several questions remain. The extent of the N availability needs to be quantified. Also, initial research suggests that high P content (e.g. Ro et al. 2015) and low K content (Krounbi et al. 2020) would facilitate binding of N, suggesting that the resulting product will need blending with other materials to produce a balanced NPK BCF.
Conclusions
Here, our aim was to review the progress made towards producing BCF with superior NUE and, in doing so, dispel misconceptions and highlight most promising venues for further research and development. With this precise question in mind, we have challenged the paradigm of producing concentrated-N slow-release BFC based on the intrinsic sorption properties of biochar. This is not to say that biochar sorption properties cannot be used to catch low-concentration N in the environment, and promising applications in such directions have been reviewed in other works. Neither do we challenge the fact that biochar products can present multiple soil benefits, thereby resulting in increased yield, as also demonstrated in numerous studies and reviews. We point however to the fact that the positive reported effects of BCFs are neither fully demonstrated in terms of N retention and slow release nor backed by ionic sorption theory. Positive effects, notably reported for commercial BCFs with low content of biochar, have been attributed to pyrolysis-derived biostimulants. With respect to boosting N retention and slow release properties, it is uncertain which technology will emerge from the current flurry of R&D activities on BCFs. Still, our review points towards some particularly promising technologies: 1) sorption of NH3 gas on specifically activated biochar, 2) synergies between biochar and clay porosities, which might provide economical enhancement of properties, 3) physical protection of solid N forms, which might be a better venue that solute sorption for highly N-concentrated BCFs. Whether a BCF delivering synergistic effects on slow-release and NUE can be produced using such methods at competitive prices, including C-credits discounts, is still unclear, but remains a cornerstone question for large-scale implementation of biochar in agriculture.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- BCF:
-
Biochar-based Fertilizer
- BC:
-
Biochar
- BCac:
-
Activated Biochar
- BCun:
-
Unmodified biochar
- BCen:
-
Enhanced biochar
- N:
-
Nitrogen
- P:
-
Phosphorus
- K:
-
Potassium
- S:
-
Sulphur
- CEC:
-
Cation Exchange Capacity
- AEC:
-
Anion exchange capacity
- NUE:
-
Nitrogen Use Efficiency
- SOM:
-
Soil Organic Matter
- DOC:
-
Dissolved Organic Carbon
- NH4 + :
-
Ammonium
- NO3 − :
-
Nitrate
References
Abollino O, Giacomino A, Malandrino M, Mentasti E (2008) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38:227–236. https://doi.org/10.1016/j.clay.2007.04.002
Amendola C, Montagnoli A, Terzaghi M, Trupiano D, Oliva F, Baronti S, Miglietta F, Chiatante D, Scippa GS (2017) Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric Ecosyst Environ 239:236–245. https://doi.org/10.1016/j.agee.2017.01.025
Amin A (2020) Carbon sequestration, kinetics of ammonia volatilization and nutrient availability in alkaline sandy soil as a function on applying calotropis biochar produced at different pyrolysis temperatures. Sci Total Environ 726:138489. https://doi.org/10.1016/j.scitotenv.2020.138489
Asada T, Ohkubo T, Kawata K, Oikawa K (2006) Ammonia adsorption on bamboo charcoal with acid treatment. J Health Sci 52:585–589. https://doi.org/10.1248/jhs.52.585
Basso AS, Miguez FE, Laird DA, Horton R, Westgate M (2013) Assessing potential of biochar for increasing water-holding capacity of sandy soils. Glob Change Biol Bioenergy 5:132–143. https://doi.org/10.1111/gcbb.12026
Blackwell P, Joseph S, Munroe P, Anawar HM, Storer P, Gilkes RJ, Solaiman ZM (2015) Influences of biochar and biochar-mineral complex on mycorrhizal colonisation and nutrition of wheat and Sorghum. Pedosphere 25:686–695. https://doi.org/10.1016/S1002-0160(15)30049-7
Borchard N, Schirrmann M, Cayuela ML, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizábal T, Sigua G, Spokas K, Ippolito JA, Novak J (2019) Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Total Environ 651:2354–2364. https://doi.org/10.1016/j.scitotenv.2018.10.060
Brewer CE, Chuang VJ, Masiello CA, Gonnermann H, Gao XD, Dugan B, Driver LE, Panzacchi P, Zygourakis K, Davies CA (2014) New approaches to measuring biochar density and porosity. Biomass Bioenergy 66:176–185. https://doi.org/10.1016/j.biombioe.2014.03.059
Brussaard L, de Ruiter PC, Brown GG (2007) Soil biodiversity for agricultural sustainability. Agric Ecosyst Environ 121:233–244. https://doi.org/10.1016/j.agee.2006.12.013
Budai A, Wang L, Gronli M, Strand LT, Antal MJ, Abiven S, Dieguez-Alonso A, Anca-Couce A, Rasse DP (2014) Surface properties and chemical composition of corncob and Miscanthus biochars: effects of production temperature and method. J Agric Food Chem 62:3791–3799. https://doi.org/10.1021/jf501139f
Budai A, Rasse DP, Lagomarsino A, Lerch TZ, Paruch L (2016) Biochar persistence, priming and microbial responses to pyrolysis temperature series. Biol Fertil Soils 52:749–761. https://doi.org/10.1007/s00374-016-1116-6
Cai Y, Qi H, Liu Y, He X (2016) Sorption/desorption behavior and mechanism of NH4+ by biochar as a nitrogen fertilizer sustained-release material. J Agric Food Chem 64:4958–4964. https://doi.org/10.1021/acs.jafc.6b00109
Carey DE, McNamara PJ, Zitomer DH (2015) Biochar from pyrolysis of biosolids for nutrient adsorption and turfgrass cultivation. Water Environ Res 87:2098–2106. https://doi.org/10.2175/106143015X14362865227391
Carneiro JSS, Lustosa Filho JF, Nardis BO, Ribeiro-Soares J, Zinn YL, Melo LCA (2018) Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain Chem Eng 6:14203–14212. https://doi.org/10.1021/acssuschemeng.8b02841
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.009
Chen L, Chen XL, Zhou CH, Yang HM, Ji SF, Tong DS, Zhong ZK, Yu WH, Chu MQ (2017) Environmental-friendly montmorillonite-biochar composites: facile production and tunable adsorption-release of ammonium and phosphate. J Clean Prod 156:648–659. https://doi.org/10.1016/j.jclepro.2017.04.050
Chen W, Meng J, Han X, Lan Y, Zhang W (2019) Past, present, and future of biochar. Biochar 1:75–87. https://doi.org/10.1007/s42773-019-00008-3
Chew J, Zhu L, Nielsen S, Graber E, Mitchell DRG, Horvat J, Mohammed M, Liu M, van Zwieten L, Donne S, Munroe P, Taherymoosavi S, Pace B, Rawal A, Hook J, Marjo C, Thomas DS, Pan G, Li L et al (2020) Biochar-based fertilizer: supercharging root membrane potential and biomass yield of rice. Sci Total Environ 713:136431. https://doi.org/10.1016/j.scitotenv.2019.136431
Chia CH, Munroe P, Joseph S, Lin Y (2010) Microscopic characterization of synthetic Terra Preta. Soil Res 48:593–605. https://doi.org/10.1071/SR10012
Chia CH, Singh BP, Joseph S, Graber ER, Munroe P (2014) Characterization of an enriched biochar. J Anal Appl Pyrolysis 108:26–34. https://doi.org/10.1016/j.jaap.2014.05.021
Christidis G (2013) Assessment of industrial clays. Bergaya F, Theng BKG, Lagaly G. (Eds.), In. Developments in clay science, Elsevier B.V., Hardbound (2013), pp. 425–449 https://doi.org/10.1016/B978-0-08-098259-5.00017-2
Conte P, Laudicina V (2017) Mechanisms of organic organic coating coating on the surface surface of a poplar poplar Biocharbiochar. Curr Org Chem 21:559–565. https://doi.org/10.2174/1385272821666161216122
Conte P, Hanke UM, Marsala V, Cimò G, Alonzo G, Glaser B (2014) Mechanisms of water interaction with pore systems of hydrochar and pyrochar from poplar forestry waste. J Agric Food Chem 62:4917–4923. https://doi.org/10.1021/jf5010034
Cornelissen G, Martinsen V, Shitumbanuma V, Alling V, Breedveld GD, Rutherford DW, Sparrevik M, Hale SE, Obia A, Mulder J (2013) Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 3:256–274. https://doi.org/10.3390/agronomy3020256
Cui X, Hao H, Zhang C, He Z, Yang X (2016) Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars. Sci Total Environ 539:566–575. https://doi.org/10.1016/j.scitotenv.2015.09.022
Dong D, Wang C, Van Zwieten L, Wang H, Jiang P, Zhou M, Wu W (2019) An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J Soils Sediments 1-14. https://doi.org/10.1007/s11368-019-02401-8
Duan W, Oleszczuk P, Pan B, Xing B (2019) Environmental behavior of engineered biochars and their aging processes in soil. Biochar 1:339–351. https://doi.org/10.1007/s42773-019-00030-5
El Sharkawi HM, Tojo S, Chosa T, Malhat FM, Youssef AM (2018) Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenergy 117:154–160. https://doi.org/10.1016/j.biombioe.2018.07.007
European Commission Implementing Regulation (EU) 2019/2164: Amending Regulation (EC) No 889/2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Regulation (EU) 2019/2164 (2019). Available online: http://eur-lex.europa.eu/eli/reg_impl/2019/2164
European Commission Regulation (EU) 2019/1009: Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (2019) (EC) No 2003/2003 Available online: http://eur-lex.europa.eu/eli/reg/2019/1009
Fageria NK, Baligar VC (2005) Enhancing nitrogen use efficiency in crop plants. Adv Agron 88:97–185. https://doi.org/10.1016/S0065-2113(05)88004-6
Farrar MB, Wallace HM, Xu CY, Nguyen TTN, Tavakkoli E, Joseph S, Bai SH (2019) Short-term effects of organo-mineral enriched biochar fertiliser on ginger yield and nutrient cycling. J Soils Sediments 19:668–682. https://doi.org/10.1007/s11368-018-2061-9
Fidel RB, Laird DA, Thompson ML, Lawrinenko M (2017) Characterization and quantification of biochar alkalinity. Chemosphere 167:367–373. https://doi.org/10.1016/j.chemosphere.2016.09.151
Fidel RB, Laird DA, Spokas KA (2018) Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci Rep 8:17627. https://doi.org/10.1038/s41598-018-35534-w
Fischer D, Glaser B (2012) Synergisms between compost and biochar for sustainable soil amelioration. In: Kumar S (ed) Management of Organic Waste. In Tech, Rijeka and Shanghai, pp 167–198. https://doi.org/10.5772/31200
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 9:e113888. https://doi.org/10.1371/journal.pone.0113888
Gao F, Xue YW, Deng PY, Cheng XR, Yang K (2015) Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem Speciat Bioavailab 27:92–97. https://doi.org/10.1080/09542299.2015.1087162
GB/Z 39121-2020 (2020) National Standard Announcement no. 21 of 2020. State Administration for Market Regulation. National Standardization Administration
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The 'Terra Preta' phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41. https://doi.org/10.1007/s001140000193
González ME, Cea M, Medina J, González A, Diez MC, Cartes P, Monreal C, Navia R (2015) Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci Total Environ 505:446–453. https://doi.org/10.1016/j.scitotenv.2014.10.014
Guenet B, Gabrielle B, Chenu C, Arrouays D, Balesdent J, Bernoux M, Bruni E, Caliman J-P, Cardinael R, Chen S, Ciais P, Desbois D, Fouche J, Frank S, Henault C, Lugato E, Naipal V, Nesme T, Obersteiner M et al (2021) Can N2O emissions offset the benefits from soil organic carbon storage? Glob Change Biol 27:237–256. https://doi.org/10.1111/gcb.15342
Gwenzi W, Nyambishi TJ, Chaukura N, Mapope N (2018) Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int J Environ Sci Technol 15: 405–414. https://doi.org/10.1007/s13762-017-1399-7
Hagemann N, Harter J, Kaldamukova R, Guzman-Bustamante I, Ruser R, Graeff S, Kappler A, Behrens S (2017a) Does soil aging affect the N2O mitigation potential of biochar? A combined microcosm and field study. Glob. Change Biol. Bioenergy 9:953–964. https://doi.org/10.1111/gcbb.12390
Hagemann N, Joseph S, Schmidt H-P, Kammann CI, Harter J, Borch T, Young RB, Varga K, Taherymoosavi S, Elliott KW, McKenna A, Albu M, Mayrhofer C, Obst M, Conte P, Dieguez-Alonso A, Orsetti S, Subdiaga E, Behrens S, Kappler A (2017b) Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat Commun 8:1089. https://doi.org/10.1038/s41467-017-01123-0
Hagemann N, Kammann CI, Schmidt H-P, Kappler A, Behrens S (2017c) Nitrate capture and slow release in biochar amended compost and soil. PLoS One 12:e0171214. https://doi.org/10.1371/journal.pone.0171214
Haider G, Koyro H-W, Azam F, Steffens D, Müller C, Kammann C (2015) Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 395:141–157. https://doi.org/10.1007/s11104-014-2294-3
Hale SE, Alling V, Martinsen V, Mulder J, Breedveld G, Cornelissen G (2013) The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 91:1612–1619. https://doi.org/10.1016/j.chemosphere.2012.12.057
Harter J, Krause H-M, Schuettler S, Ruser R, Fromme M, Scholten T, Kappler A, Behrens S (2013) Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J 8:660. https://doi.org/10.1038/ismej.2013.160
He Y, Zhou X, Jiang L, Li M, Du Z, Zhou G, Shao J, Wang X, Xu Z, Hosseini Bai S, Wallace H, Xu C (2017) Effects of biochar application on soil greenhouse gas fluxes: a meta-analysis. Glob. Change Biol. Bioenergy 9:743–755. https://doi.org/10.1111/gcbb.12376
Heaney N, Ukpong E, Lin C (2020) Low-molecular-weight organic acids enable biochar to immobilize nitrate. Chemosphere 240:124872. https://doi.org/10.1016/j.chemosphere.2019.124872
Hestrin R, Torres-Rojas D, Dynes JJ, Hook JM, Regier TZ, Gillespie AW, Smernik RJ, Lehmann J (2019) Fire-derived organic matter retains ammonia through covalent bond formation. Nat Commun 10:664. https://doi.org/10.1038/s41467-019-08401-z
Hina K, Hedley M, Camps-Arbestain M, Hanly J (2015) Comparison of pine bark, biochar and zeolite as sorbents for NH4+-N removal from water. Clean – soil. Air, Water 43:86–91. https://doi.org/10.1002/clen.201300682
Hollister CC, Bisogni JJ, Lehmann J (2013) Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn Stover (Zea mays L.) and oak wood (Quercus spp.). J Environ Qual 42:137–144. https://doi.org/10.2134/jeq2012.0033
Huang X, Bai J, Li KR, Zhao YG, Tian WJ, Hu CH (2020) Preparation of clay/biochar composite adsorption particle and performance for Ammonia nitrogen removal from aqueous solution. J Ocean Univ China 19:729–739. https://doi.org/10.1007/s11802-020-4150-9
Huff MD, Marshall S, Saeed HA, Lee JW (2018) Surface oxygenation of biochar through ozonization for dramatically enhancing cation exchange capacity. Bioresour Bioprocess 5:18. https://doi.org/10.1186/s40643-018-0205-9
Hyvaluoma J, Hannula M, Arstila K, Wang HL, Kulju S, Rasa K (2018) Effects of pyrolysis temperature on the hydrologically relevant porosity of willow biochar. J Anal Appl Pyrolysis 134:446–453. https://doi.org/10.1016/j.jaap.2018.07.011
Ibrahim MM, Tong C, Hu K, Zhou B, Xing S, Mao Y (2020) Biochar-fertilizer interaction modifies N-sorption, enzyme activities and microbial functional abundance regulating nitrogen retention in rhizosphere soil. Sci Total Environ 739:140065. https://doi.org/10.1016/j.scitotenv.2020.140065
Ismadji S, Tong DS, Soetaredjo FE, Ayucitra A, Yu WH, Zhou CH (2016) Bentonite hydrochar composite for removal of ammonium from koi fish tank. Appl Clay Sci 119:146–154. https://doi.org/10.1016/j.clay.2015.08.022
ISO 8157:2015 Fertilizers and soil conditioners - Vocabulary, 2nd ed., International Organization for Standardization. Available online: https://www.iso.org/obp/ui/#iso:std:iso:8157:ed-2:v1:en. Accessed 3 Jan 2021
Jassal RS, Johnson MS, Molodovskaya M, Black TA, Jollymore A, Sveinson K (2015) Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J Environ Manag 152:140–144. https://doi.org/10.1016/j.jenvman.2015.01.021
Jellali S, El-Bassi L, Charabi Y, Uaman M, Khiari B, Al-Wardy M, Jeguirim M (2022) Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: a critical review on benefits for environment and agriculture. J Environ Manag 305:114368. https://doi.org/10.1016/j.jenvman.2021.114368
Jia YM, Hu ZY, Mu J, Zhang WT, Xie ZJ, Wang GX (2020) Preparation of biochar as a coating material for biochar-coated urea. Sci Total Environ 731:139063. https://doi.org/10.1016/j.scitotenv.2020.139063
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE (2010) An investigation into the reactions of biochar in soil. Aust J Soil Res 48:501–515. https://doi.org/10.1071/sr10009
Joseph S, Graber ER, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan GX, Li L, Taylor P, Rawal A, Hook J (2013) Shifting paradigms: development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manage 4:323–343. https://doi.org/10.4155/cmt.13.23
Joseph S, Anawar HM, Storer P, Blackwell P, Chia C, Lin Y, Munroe P, Donne S, Horvat J, Wang JL, Solaiman ZM (2015) Effects of enriched biochars containing magnetic Iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 25:749–760. https://doi.org/10.1016/S1002-0160(15)30056-4
Joseph S, Kammann CI, Shepherd JG, Conte P, Schmidt H-P, Hagemann N, Rich AM, Marjo CE, Allen J, Munroe P, Mitchell DRG, Donne S, Spokas K, Graber ER (2018) Microstructural and associated chemical changes during the composting of a high temperature biochar: mechanisms for nitrate, phosphate and other nutrient retention and release. Sci Total Environ 618:1210–1223. https://doi.org/10.1016/j.scitotenv.2017.09.200
Kameyama K, Iwata Y, Miyamoto T (2017) Biochar amendment of soils according to their physicochemical properties. Jpn Agric Res Q : JARQ 51:117–127. https://doi.org/10.6090/jarq.51.117
Kammann C, Ratering S, Eckhard C, Müller C (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41:1052–1066. https://doi.org/10.2134/jeq2011.0132
Kammann C, Schmidt H-P, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro H-W, Conte P, Joseph S (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep 5:11080. https://doi.org/10.1038/srep11080
Kang S, Jung J, Choe JK, Ok YS, Choi Y (2018) Effect of biochar particle size on hydrophobic organic compound sorption kinetics: applicability of using representative size. Sci Total Environ 619:410–418. https://doi.org/10.1016/j.scitotenv.2017.11.129
Keskinen R, Nikama J, Kaseva J, Rasa K (2021) Feasibility of nitrogen-enriched chars as circular fertilizers. Waste Biomass Valorization 12:6823–6833. https://doi.org/10.1007/s12649-021-01471-5
Khajavi-Shojaei S, Moezzi A, Norouzi Masir M, Taghavi M (2020) Synthesis modified biochar-based slow-release nitrogen fertilizer increases nitrogen use efficiency and corn (Zea mays L.) growth. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01137-7
Khan N, Clark I, Sánchez-Monedero MA, Shea S, Meier S, Qi F, Kookana RS, Bolan N (2016) Physical and chemical properties of biochars co-composted with biowastes and incubated with a chicken litter compost. Chemosphere 142:14–23. https://doi.org/10.1016/j.chemosphere.2015.05.065
Kizito S, Wu S, Kipkemoi Kirui W, Lei M, Lu Q, Bah H, Dong R (2015) Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci Total Environ 505:102–112. https://doi.org/10.1016/j.scitotenv.2014.09.096
Kocatürk-Schumacher NP, Bruun S, Zwart K, Jensen LS (2017a) Nutrient recovery from the liquid fraction of digestate by clinoptilolite. Clean-Soil, Air, Water 45:1500153. https://doi.org/10.1002/clen.201500153
Kocatürk-Schumacher NP, Zwart K, Bruun S, Brussaard L, Jensen LS (2017b) Does the combination of biochar and clinoptilolite enhance nutrient recovery from the liquid fraction of biogas digestate? Environ Technol 38:1313–1323. https://doi.org/10.1080/09593330.2016.1226959
Kocatürk-Schumacher NP, Zwart K, Bruun S, Stoumann Jensen L, Sørensen H, Brussaard L (2019) Recovery of nutrients from the liquid fraction of digestate: use of enriched zeolite and biochar as nitrogen fertilizers. J Plant Nutr Soil Sci 182:187–195. https://doi.org/10.1002/jpln.201800271
Kolton M, Graber ER, Tsehansky L, Elad Y, Cytryn E (2017) Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol 213:1393–1404. https://doi.org/10.1111/nph.14253
Koon JH, Kaufman WJ (1975) Ammonia removal from municipal wastewaters by ion-exchange. J Water Pollut Control Fed 47: 448–465. http://www.jstor.org/stable/25038654
Krounbi L, Enders A, Anderton CR, Engelhard MH, Hestrin R, Torres-Rojas D, Dynes JJ, Lehmann J (2020) Sequential ammonia and carbon dioxide adsorption on pyrolyzed biomass to recover waste stream nutrients. ACS Sustain Chem Eng 8:7121–7131. https://doi.org/10.1021/acssuschemeng.0c01427
Krounbi L, Enders A, Gaunt J, Ball M, Lehmann J (2021) Plant uptake of nitrogen adsorbed to biochars made from dairy manure. Sci Rep 11:15001. https://doi.org/10.1038/s41598-021-94337-8
Laird DA, Fleming P, Davis DD, Horton R, Wang BQ, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449. https://doi.org/10.1016/j.geoderma.2010.05.013
Lawrinenko M, Laird DA (2015) Anion exchange capacity of biochar. Green Chem 17:4628–4636. https://doi.org/10.1039/C5GC00828J
Lawrinenko M, Laird DA, Johnson RL, Jing D (2016) Accelerated aging of biochars: impact on anion exchange capacity. Carbon 103:217–227. https://doi.org/10.1016/j.carbon.2016.02.096
Lehmann J (2007) A handful of carbon. Nature 447:143. https://doi.org/10.1038/447143a
Lehmann J, da Silva Jr JP, Rondon M, Cravo MdS, Greenwood J, Nehls T, Steiner C, Glaser B (2002) Slash-and-char-a feasible alternative for soil fertility management in the central Amazon. Proceedings of the 17th World Congress of Soil Science. Soil Fert. Soc. of Thailand Bangkok
Li PJ, Lin KR, Fang ZQ, Wang KM (2017) Enhanced nitrate removal by novel bimetallic Fe/Ni nanoparticles supported on biochar. J Clean Prod 151:21–33. https://doi.org/10.1016/j.jclepro.2017.03.042
Li S, Harris S, Anandhi A, Chen G (2019) Predicting biochar properties and functions based on feedstock and pyrolysis temperature: a review and data syntheses. J Clean Prod 215:890–902. https://doi.org/10.1016/j.jclepro.2019.01.106
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730. https://doi.org/10.2136/sssaj2005.0383
Liang B, Chung-Ho W, Solomon D, Kinyangi J, Luizao FJ, Wirick S, Skjemstad JO, Lehmann J (2013) Oxidation is key for black carbon surface functionality and nutrient retention in Amazon Anthrosols. Br J Environ Clim Change 3:9. https://doi.org/10.9734/BJECC/2013/2267
Liao J, Liu X, Hu A, Song H, Chen X, Zhang Z (2020) Effects of biochar-based controlled release nitrogen fertilizer on nitrogen-use efficiency of oilseed rape (Brassica napus L.). Sci Rep 10:11063. https://doi.org/10.1038/s41598-020-67528-y
Lin Y, Munroe P, Joseph S, Henderson R, Ziolkowski A (2012) Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 87:151–157. https://doi.org/10.1016/j.chemosphere.2011.12.007
Lin Y, Munroe P, Joseph S, Ziolkowski A, van Zwieten L, Kimber S, Rust J (2013) Chemical and structural analysis of enhanced biochars: thermally treated mixtures of biochar, chicken litter, clay and minerals. Chemosphere 91:35–40. https://doi.org/10.1016/j.chemosphere.2012.11.063
Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:235–240. https://doi.org/10.1016/j.biortech.2012.06.085
Liu Q, Liu B, Zhang Y, Hu T, Lin Z, Liu G, Wang X, Ma J, Wang H, Jin H (2019a) Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: options and mitigation strength in a global perspective. Glob Chang Boil 25:2077–2093. https://doi.org/10.1111/gcb.14613
Liu X, Liao J, Song H, Yang Y, Guan C, Zhang Z (2019b) A biochar-based route for environmentally friendly controlled release of nitrogen: urea-loaded biochar and bentonite composite. Sci Rep 9:9548. https://doi.org/10.1038/s41598-019-46065-3
Liu C, Sun B, Zhang X, Liu X, Drosos M, Li L, Pan G (2020a) The water-soluble pool in biochar dominates maize plant growth promotion under biochar amendment. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10203-3
Liu X, Wang H, Liu C, Sun B, Zheng J, Bian R, Drosos M, Zhang X, Li L, Pan G (2020b) Biochar increases maize yield by promoting root growth in the rainfed region. Arch Agron Soil Sci 1-14. https://doi.org/10.1080/03650340.2020.1796981
Lonappan L, Liu Y, Rouissi T, Brar SK, Surampalli RY (2020) Development of biochar-based green functional materials using organic acids for environmental applications. J Clean Prod 244:118841. https://doi.org/10.1016/j.jclepro.2019.118841
Lou Y, Joseph S, Li L, Graber ER, Liu X, Pan G (2016) Water extract from straw biochar used for plant growth promotion: an initial test. BioRes. 11:249–266
Lychuk TE, Izaurralde RC, Hill RL, McGill WB, Williams JR (2015) Biochar as a global change adaptation: predicting biochar impacts on crop productivity and soil quality for a tropical soil with the environmental policy integrated climate (EPIC) model. Mitig Adapt Strateg Glob Chang 20:1437–1458. https://doi.org/10.1007/s11027-014-9554-7
Magrini-Bair KA, Czernik S, Pilath HM, Evans RJ, Maness PC, Leventhal J (2009) Biomass derived, carbon sequestering, designed fertilizers. Annals Environ Sci 3:217–225
Manikandan A, Subramanian K (2013) Urea intercalated biochar—a slow release fertilizer production and characterisation. Indian J. Sci. Technol. 6: 5579-5584. https://doi.org/10.17485/ijst/2013/v6i12.11
Mao JD, Johnson RL, Lehmann J, Olk DC, Neves EG, Thompson ML, Schmidt-Rohr K (2012) Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration. Environ Sci Technol 46:9571–9576. https://doi.org/10.1021/es301107c
Mia S, Dijkstra FA, Singh B (2017) Aging induced changes in biochar's functionality and adsorption behavior for phosphate and ammonium. Environ Sci Technol 51:8359–8367. https://doi.org/10.1021/acs.est.7b00647
Montégut G, Michelin L, Brendlé J, Lebeau B, Patarin J (2016) Ammonium and potassium removal from swine liquid manure using clinoptilolite, chabazite and faujasite zeolites. J Environ Manag 167:147–155. https://doi.org/10.1016/j.jenvman.2015.11.027
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163:247–255. https://doi.org/10.1016/j.geoderma.2011.04.021
Munera-Echeverri JL, Martinsen V, Strand LT, Zivanovic V, Cornelissen G, Mulder J (2018) Cation exchange capacity of biochar: an urgent method modification. Sci Total Environ 642:190–197. https://doi.org/10.1016/j.scitotenv.2018.06.017
Nguyen LH, Vu TM, Le TT, Trinh VT, Tran TP, Van HT (2019) Ammonium removal from aqueous solutions by fixed-bed column using corncob-based modified biochar. Environ Technol 40:683–692. https://doi.org/10.1080/09593330.2017.1404134
Nielsen S, Minchin T, Kimber S, van Zwieten L, Gilbert J, Munroe P, Joseph S, Thomas T (2014) Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric Ecosyst Environ 191:73–82. https://doi.org/10.1016/j.agee.2014.04.006
NY/T 3041-2016 Biochar-based fertilizer, People's Republic of China agricultural industry standards. Ministry of Agriculture of the People’s Republic of China (2016). Available online: http://bbs.biaozhuns.com/forum.php?mod=viewthread&tid=174155&highlight=3041-2016
NY/T 3618-2020 Biochar-based organic fertilizer, People's Republic of China agricultural industry standards. Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2020). Available online: http://bbs.biaozhuns.com/forum.php?mod=viewthread&tid=268982&highlight=3618-2020
Obia A, Mulder J, Martinsen V, Cornelissen G, Børresen T (2016) In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Tillage Res 155:35–44. https://doi.org/10.1016/j.still.2015.08.002
Omondi MO, Xia X, Nahayo A, Liu X, Korai PK, Pan G (2016) Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 274:28–34. https://doi.org/10.1016/j.geoderma.2016.03.029
O'Toole A, Moni C, Weldon S, Schols A, Carnol M, Bosman B, Rasse DP (2018) Miscanthus biochar had limited effects on soil physical properties, microbial biomass, and grain yield in a four-year field experiment in Norway. Agriculture. 8. https://doi.org/10.3390/agriculture8110171
Pan G, Bian R, Cheng K (2017) From biowaste treatment to novel bio-material manufacturing: biomaterial science and technology based on biomass pyrolysis. Sci Technol Rev 35:82–93
Park MH, Jeong S, Kim JY (2019) Adsorption of NH3-N onto rice straw-derived biochar. J Environ Chem Eng 7:103039. https://doi.org/10.1016/j.jece.2019.103039
Paulot F, Jacob DJ, Pinder RW, Bash JO, Travis K, Henze DK (2014) Ammonia emissions in the United States, European Union, and China derived by high-resolution inversion of ammonium wet deposition data: interpretation with a new agricultural emissions inventory (MASAGE_NH3). J Geophysical Res: Atmospheres 119:4343–4364. https://doi.org/10.1002/2013JD021130
Petrovic JT, Stojanovic MD, Milojkovic JV, Petrovic MS, Sostaric TD, Lausevic MD, Mihajlovic ML (2016) Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution. J Environ Manag 182:292–300. https://doi.org/10.1016/j.jenvman.2016.07.081
Portejoie S, Dourmad JY, Martinez J, Lebreton Y (2004) Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest Prod Sci 91:45–55. https://doi.org/10.1016/j.livprodsci.2004.06.013
Prost K, Borchard N, Siemens J, Kautz T, Séquaris J-M, Möller A, Amelung W (2013) Biochar affected by composting with farmyard manure. J Environ Qual 42. https://doi.org/10.2134/jeq2012.0064
Puga AP, Grutzmacher P, Cerri CEP, Ribeirinho VS, de Andrade CA (2020) Biochar-based nitrogen fertilizers: greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci Total Environ 704:135375. https://doi.org/10.1016/j.scitotenv.2019.135375
Qian L, Chen B (2014) Interactions of aluminum with biochars and oxidized biochars: implications for the biochar aging process. J Agric Food Chem 62:373–380. https://doi.org/10.1021/jf404624h
Qian L, Chen L, Joseph S, Pan G, Li L, Zheng J, Zhang X, Zheng J, Yu X, Wang J (2014) Biochar compound fertilizer as an option to reach high productivity but low carbon intensity in rice agriculture of China. Carbon Manag 5:145–154. https://doi.org/10.1080/17583004.2014.912866
Rawal A, Joseph SD, Hook JM, Chia CH, Munroe PR, Donne S, Lin Y, Phelan D, Mitchell DR, Pace B (2016) Mineral–biochar composites: molecular structure and porosity. Environ Sci Technol 50:7706–7714. https://doi.org/10.1021/acs.est.6b00685
Razzaghi F, Obour PB, Arthur E (2020) Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 361:114055. https://doi.org/10.1016/j.geoderma.2019.114055
Ro KS, Lima IM, Reddy GB, Jackson MA, Gao B (2015) Removing gaseous NH3 using biochar as an adsorbent. Agriculture 5:991–1002. https://doi.org/10.3390/agriculture5040991
Sarkhot DV, Ghezzehei TA, Berhe AA (2013) Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J Environ Qual 42:1545–1554. https://doi.org/10.2134/jeq2012.0482
Schmidt HP, Pandit BH, Martinsen V, Cornelissen G, Conte P, Kammann CI (2015) Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture 5:723–741. https://doi.org/10.3390/agriculture5030723
Schmidt HP, Pandit BH, Cornelissen G, Kammann C (2017) Biochar-based fertilization with liquid nutrient enrichment: 21 field trials covering 13 crop species in Nepal. Land Degrad Dev 28:2324–2342. https://doi.org/10.1002/ldr.2761
Shi W, Ju Y, Bian R, Li L, Joseph S, Mitchell DRG, Munroe P, Taherymoosavi S, Pan G (2020) Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci Total Environ 701:134424. https://doi.org/10.1016/j.scitotenv.2019.134424
Shi W, Bian R, Li L, Lian W, Liu X, Zheng J, Cheng K, Zhang X, Drosos M, Joseph S, Pan G (2022) Assessing the impacts of biochar-blended urea on nitrogen use efficiency and soil retention in wheat production. GCB Bioenergy 14:65–83. https://doi.org/10.1111/gcbb.12904
Silber A, Levkovitch I, Graber ER (2010) pH-dependent mineral release and surface properties of Cornstraw biochar: agronomic implications. Environ Sci Technol 44:9318–9323. https://doi.org/10.1021/es101283d
Sim DHH, Tan IAW, Lim LLP, Hameed BH (2021) Encapsulated biochar-based sustained release fertilizer for precision agriculture: a review. J Clean Prod 303:127018. https://doi.org/10.1016/j.jclepro.2021.127018
Singh SV, Chaturvedi S, Dhyani VC, Kasivelu G (2020) Pyrolysis temperature influences the characteristics of rice straw and husk biochar and sorption/desorption behaviour of their biourea composite. Bioresour Technol 314:123674. https://doi.org/10.1016/j.biortech.2020.123674
Sizmur T, Fresno T, Akgül G, Frost H, Moreno-Jiménez E (2017) Biochar modification to enhance sorption of inorganics from water. Bioresour Technol 246:34–47. https://doi.org/10.1016/j.biortech.2017.07.082
Soussana JF, Lutfalla S, Smith P, Lal R, Chenu C, Ciais P (2017) Letter to the editor: answer to the viewpoint "sequestering soil organic carbon: a nitrogen dilemma". Environ Sci Technol 51:11502. https://doi.org/10.1021/acs.est.7b03932
Suliman W, Harsh JB, Abu-Lail NI, Fortuna A-M, Dallmeyer I, Garcia-Perez M (2016) Modification of biochar surface by air oxidation: role of pyrolysis temperature. Biomass Bioenergy 85:1–11. https://doi.org/10.1016/j.biombioe.2015.11.030
Sun J, Zheng J, Cheng K, Ye Y, Zhuang Y, Pan G (2018) Quantifying carbon sink by biochar compound fertilizer project for domestic voluntary carbon trading in agriculture. Scientia Agric Sinica 51:4470–4484. https://doi.org/10.3864/j.issn.0578-1752.2018.23.007
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69. https://doi.org/10.1007/s11104-011-0870-3
Takaya CA, Fletcher LA, Singh S, Anyikude KU, Ross AB (2016) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527. https://doi.org/10.1016/j.chemosphere.2015.11.052
van Groenigen JW, van Kessel C, Hungate BA, Oenema O, Powlson DS, van Groenigen KJ (2017) Sequestering soil organic carbon: a nitrogen dilemma. Environ Sci Technol 51:4738–4739. https://doi.org/10.1021/acs.est.7b01427
Viglašová E, Galamboš M, Danková Z, Krivosudský L, Lengauer CL, Hood-Nowotny R, Soja G, Rompel A, Matík M, Briančin J (2018) Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag 79:385–394. https://doi.org/10.1016/j.wasman.2018.08.005
Wang B, Lehmann J, Hanley K, Hestrin R, Enders A (2015) Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 138:120–126. https://doi.org/10.1016/j.chemosphere.2015.05.062
Wang B, Lehmann J, Hanley K, Hestrin R, Enders A (2016) Ammonium retention by oxidized biochars produced at different pyrolysis temperatures and residence times. RSC Adv 6:41907–41913. https://doi.org/10.1039/c6ra06419a
Wang Z, Li J, Zhang G, Zhi Y, Yang D, Lai X, Ren T (2020) Characterization of acid-aged biochar and its ammonium adsorption in an aqueous solution. Materials (Basel) 13:2270. https://doi.org/10.3390/ma13102270
Wang M, Xiang A, Gao Z, Zhang K, Ren Y, Hu Z (2021) Study on the nitrogen-releasing characteristics and mechanism of biochar-based urea infiltration fertilizer. Biomass Convers Biorefin:1–11
Weldon S, Rasse DP, Budai A, Tomic O, Dörsch P (2019) The effect of a biochar temperature series on denitrification: which biochar properties matter? Soil biol. Biochem. 135:173–183. https://doi.org/10.1016/j.soilbio.2019.04.018
Wen P, Wu Z, Han Y, Cravotto G, Wang J, Ye B-C (2017) Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain Chem Eng 5:7374–7382. https://doi.org/10.1021/acssuschemeng.7b01721
Wiedner K, Fischer D, Walther S, Criscuoli I, Favilli F, Nelle O, Glaser BJ (2015) Acceleration of biochar surface oxidation during composting? J Agric Food Chem 63(15):3830–3837. https://doi.org/10.1021/acs.jafc.5b00846
Wong JWC, Webber JBW, Ogbonnaya UO (2019) Characteristics of biochar porosity by NMR and study of ammonium ion adsorption. J Anal Appl Pyrolysis 143:104687. https://doi.org/10.1016/j.jaap.2019.104687
Wu WX, Yang M, Feng QB, McGrouther K, Wang HL, Lu HH, Chen YX (2012) Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 47:268–276. https://doi.org/10.1016/j.biombioe.2012.09.034
Xiang Y, Deng Q, Duan H, Guo Y (2017) Effects of biochar application on root traits: a meta-analysis. Glob Change Biol Bioenergy 9:1563–1572. https://doi.org/10.1111/gcbb.12449
Xiang AH, Qi RY, Wang MF, Zhang K, Jiang EC, Ren YZ, Hu ZW (2020) Study on the infiltration mechanism of molten urea and biochar for a novel fertilizer preparation. Ind Crop Prod 153:112558. https://doi.org/10.1016/j.indcrop.2020.112558
Xu P, Koloutsou-Vakakis S, Rood MJ, Luan S (2017) Projections of NH3 emissions from manure generated by livestock production in China to 2030 under six mitigation scenarios. Sci Tot Environ 607-608:78–86. https://doi.org/10.1016/j.scitotenv.2017.06.258
Xue YW, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, Ro KS (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200:673–680. https://doi.org/10.1016/j.cej.2012.06.116
Xue LH, Gao B, Wan YS, Fang JN, Wang SS, Li YC, Munoz-Carpena R, Yang LZ (2016) High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J Taiwan Inst Chem Eng 63:312–317. https://doi.org/10.1016/j.jtice.2016.03.021
Yan M, Pan G, Lavallee JM, Conant RT (2020) Rethinking sources of nitrogen to cereal crops. Glob Chang Biol 26:191–199. https://doi.org/10.1111/gcb.14908
Yao Y, Gao B, Fang J, Zhang M, Chen H, Zhou Y, Creamer AE, Sun Y, Yang L (2014) Characterization and environmental applications of clay–biochar composites. Chem Eng J 242:136–143. https://doi.org/10.1016/j.cej.2013.12.062
Yao C, Joseph S, Li L, Pan G, Lin Y, Munroe P, Pace B, Taherymoosavi S, Van Zwieten L, Thomas T, Nielsen S, Ye J, Donne S (2015) Developing more effective enhanced biochar fertilisers for improvement of pepper yield and quality. Pedosphere 25:703–712. https://doi.org/10.1016/S1002-0160(15)30051-5
Ye J, Zhang R, Nielsen S, Joseph SD, Huang D, Thomas T (2016) A combination of biochar–mineral complexes and compost improves soil bacterial processes, soil quality, and plant properties. Front Microbiol 7:372. https://doi.org/10.3389/fmicb.2016.00372
Ye L, Camps-Arbestain M, Shen Q, Lehmann J, Singh B, Sabir M (2020) Biochar effects on crop yields with and without fertilizer: a meta-analysis of field studies using separate controls. Soil Use Manag 36:2–18. https://doi.org/10.1111/sum.12546
Zeng Z, Zhang SD, Li TQ, Zhao FL, He ZL, Zhao HP, Yang XE, Wang HL, Zhao J, Rafiq MT (2013) Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J Zhejiang Univ Sci B 14:1152–1161. https://doi.org/10.1631/jzus.B1300102
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528:51–59. https://doi.org/10.1038/nature15743
Zhang D, Pan G, Wu G, Kibue GW, Li L, Zhang X, Zheng J, Zheng J, Cheng K, Joseph S, Liu X (2016) Biochar helps enhance maize productivity and reduce greenhouse gas emissions under balanced fertilization in a rainfed low fertility inceptisol. Chemosphere 142:106–113. https://doi.org/10.1016/j.chemosphere.2015.04.088
Zhang J, Hu X, Yan J, Long L, Xue Y (2020a) Crayfish shell biochar modified with magnesium chloride and its effect on lead removal in aqueous solution. Environ Sci Pollut Res Int 27:9582–9588. https://doi.org/10.1007/s11356-020-07631-9
Zhang M, Song G, Gelardi DL, Huang L, Khan E, Mašek O, Parikh SJ, Ok YS (2020b) Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res 186:116303. https://doi.org/10.1016/j.watres.2020.116303
Zheng H, Wang ZY, Deng X, Herbert S, Xing BS (2013) Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 206:32–39. https://doi.org/10.1016/j.geoderma.2013.04.018
Zheng J, Han J, Liu Z, Xia W, Zhang X, Li L, Liu X, Bian R, Cheng K, Zheng J, Pan G (2017) Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric Ecosys Environ 241:70–78. https://doi.org/10.1016/j.agee.2017.02.034
Zwart K (2020) Effects of Biochar Produced from Waste on Soil Quality. Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste: 283-299. https://doi.org/10.1002/9781118921487
Acknowledgements
This work was supported by the Research Council of Norway through the Carbo-Fertil project NFR281113. C. Kammann gratefully acknowledges the support of the FACCE-JPI project “ABC4Soil” via grant no. 031B0588B.
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and internal review of this manuscript. Conceptualization: D.P. Rasse, S Weldon and N.P. Kocatürk-Schumacher. Collection of published data: S. Weldon, A. O’Toole and N.P. Kocatürk-Schumacher. Harmonization and in-depth editing: D.P. Rasse.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflict of interest/competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Ismail Cakmak.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 314 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasse, D.P., Weldon, S., Joner, E.J. et al. Enhancing plant N uptake with biochar-based fertilizers: limitation of sorption and prospects. Plant Soil 475, 213–236 (2022). https://doi.org/10.1007/s11104-022-05365-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05365-w