Abstract
Purpose
Charred materials are low in bioavailable nitrogen (N) due to gaseous losses and the formation of recalcitrant structures during pyrolysis. Enriching chars with N from wastewaters offers a possibility to upgrade the agronomic value of the chars and manage the liquids. For assessing the practical feasibility of the approach, more information on the extent of the retention and release of the loaded N is needed.
Methods
The ammonium-N (NH4-N) retention capacity of chars derived from sewage sludge (SS_A-C), Salix wood (SA), broiler manure (BR) and coal (LG85) was determined via equilibrations in solutions containing 400, 1500 and 5000 mg NH4-N L−1. Plant availability of the loaded N in SS_C, SA and BR was studied in a pot experiment with ryegrass.
Results
Differences in the total N retention of moist chars were small. The amount of N retained increased with increase in the solution N and was at the highest 2–4 g NH4-N L−1 char. In four consecutive ryegrass harvests, the apparent N recoveries were 67, 47 and 34% for SA, BR and SS_C treatments. No slow release of N was observed.
Conclusion
Considering crop production, the amounts of N retained within the studied chars in bioavailable form were small. Chars with a higher N retention capacity would be needed for an efficient cascade from water purification to fertilizer use.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The NH4-N sorption capacity of various charred materials has been studied quite extensively from a theoretical point of view to appreciate the potential of chars in treating N-rich wastewaters. The produced N enriched chars are thought to serve as slow-release N fertilizers but studies on their real fertilizer value are scarce. This study provides a practically oriented research on the retention and release of NH4-N loaded from strong N solutions into chars derived from various feedstocks. The paper highlights key issues that need to be considered and resolved before the approach can be put into practice.
Introduction
Chars for soil amendment can be produced from various organic waste and side stream biomasses by heating in low oxygen conditions [1, 2]. The pyrolysis process is advantageous in that it reduces the weight and volume of the biomass for transportation, eliminates pathogens and harmful organic compounds, enhances carbon (C) sequestration, and yields energy in a form of upgradable gas [3,4,5]. Agronomic benefits achievable by char amendments in soil comprise improved water holding ability, increased cation exchange capacity (CEC) and nutrient availability, and higher pH values [6, 7]. The key properties of chars contributing to these possible positive impacts, namely porosity, surface activity and nutrient contents vary greatly depending on the process conditions and especially on the feedstock material [e.g., 8–11]. Chars derived from animal manures and biosolids are generally rich in nutrient elements due to the inherently high nutrient contents of the feedstock biomasses, whereas wood-based (lignocellulosic) chars typically contain relatively low amounts of nutrients but exhibit the greatest C contents [12]. However, scarcity of bioavailable nitrogen (N), is common to all charred materials due to gaseous losses of N compounds and the formation of recalcitrant N-ring structures during pyrolysis [13,14,15,16,17]. An increase in the maximum process temperature tends to increase volatilization of N and other volatile elements (e.g., C, and sulfur (S)), whereas non-volatile elements (e.g., phosphorus (P) and potassium (K)) remain enriched in the char fraction [9, 18].
Increasing the N content of charred materials after pyrolysis is one possibility to upgrade the agronomic value of the product since N is the nutrient most often limiting plant growth. Chars derived from straw, shells, manure and woody materials have shown to sorb N from aqueous solutions, especially in the ammonium (NH4+) form, though the range of sorption capacity extends from below 1 to several dozens of mg N per g char [e.g. 19–25]. The char NH4-N sorption ability has been linked to the abundance of negatively charged surface functional groups which bind cations via electrostatic interactions [20, 24, 26, 27]. Furthermore, surface precipitation, complex formation, and physical entrapment of NH4+ within the char matrix are among the identified retention mechanisms [22, 28, 29]. For example, Li et al. [24] found char CEC and micropore volume to be the main factors governing NH4+ adsorption on chars derived from switchgrass, water oak and biosolids, whereas Fan et al. [28] found evidence of surface precipitation of struvite [MgNH4(PO)4] and complexation between NH4+ and surface hydroxyl species in sorption studies with bamboo biochar.
Exploiting recycled sources of N (e.g., in animal slurries, stripper effluents or other nutrient-rich process waters) in char loading would result in a circular bio-based fertilizer product that could reduce the input of new reactive N into the environment and contribute to C sequestration. Encouraging results have been achieved in enriching chars with urea ammonium nitrate [22], anaerobic digestate slurry from piggery manure [23], and dairy effluent [30]. Even though some chars seem to be able to carry agronomically meaningful amounts of N, the approach may be challenged by strong retention. Limited release of the added N to salt extracts (0.001–1 M KCl or CaCl2) [22, 26, 30] and by plant uptake [31] has been reported. However, more results on the plant availability of N loaded in chars are needed from pot and field tests involving plants to assess the usability and value of various N enriched chars in agriculture.
In this study, the NH4-N sorption capacity of six chars derived from four contrasting feedstock materials (sewage sludge, Salix wood, broiler manure and coal) was assessed in a set of laboratory equilibration experiments. Thereafter, the release of retained N from selected chars was investigated in a pot experiment with ryegrass. The aim was to determine whether N enriched chars can retain and release N in rates appropriate for crop production or landscaping and to identify the most promising raw materials for such use.
Materials and Methods
Tested Chars
Chars derived from sewage sludge, Salix wood and broiler manure via a slow pyrolysis process and a commercial activated charcoal were included in the study (Table 1). The sewage-sludges originated from wastewater treatment plants in the two largest cities of Finland, Turku and Espoo, and were processed after vacuum drying (87–97% dry matter content) by an electronically heated industrial pilot-scale pyrolysis device (Ecomation Ltd, Finland) operated continuously [32]. Salix wood was chipped and dried (91% dry matter content) before pyrolysis, which was conducted using a robust pilot-scale batch-type (batch size ca. 90 kg) reactor. The targeted process peak temperature was 450–500 °C, and the process was run until the gas formation ceased. Sphagnum peat bedded broiler manure was obtained from Biolan Oy (Eura, Finland), pre-dried at 37 °C and pyrolyzed in a batch-type laboratory-scale pyrolysis equipment [9]. Commercial activated charcoal (SORBOTECH LG85) intended for filtration use was acquired from a local distributor.
Char Nitrogen Retention Capacity
The chars were gently crushed and sieved to obtain a particle size fraction of 0.6–2 mm, which was used in N sorption experiments.
The sorption was carried out at room temperature using laboratory grade ammonium sulfate ((NH4)2SO4) solution at concentrations of 400, 1500 and 5000 mg N L−1 and pH levels of 6 and 8 adjusted with sodium hydroxide (NaOH) and sulfuric acid (H2SO4) before equilibration. The chosen pH range was expected to cover the maximum sorption potential of the chars [23, 28, 33]. At pH 6, the ratio of ammonia (NH3) to NH4+ is strongly in favor of NH4+ and very small quantities of NH3 can be expected to be released, whereas at pH 8 NH3 has a stronger role [34, 35].
According to the volume weight determined for the sieved size fraction of each char, 10 ml (1.9–7.3 g) of char was weighed and placed in a 250 mL flask, after which 100 mL of N solution was added and the suspensions were shaken for 24 h in a rotating shaker. Thereafter, the suspensions were filtered (Whatman Grade 589/3) and the filtrates were analyzed for pH (SevenCompact Duo Mettler Toledo, InLAb® Expert Pro-ISM) and NH4-N concentrations using a continuous flow analyzer (Skalar San++System). Each treatment was conducted in three replicates. The amount of N adsorbed was calculated per char volume from the difference of solution N concentrations in blank samples containing no char and char-containing samples. Volume basis was adopted due to the considerable differences in the volume weights of the studied char materials rendering mass-based comparisons less meaningful (Table 1).
In addition, the N content of the char fraction was determined to include free N occurring in the solution that was retained within the char pores and between char particles. The chars were analyzed fresh using the Kjeldahl method (in-house procedure based on SFS EN ISO 20483:2013, EN ISO 5983-2 and AOAC 2001.11; FOSS Kjeltec™ 8400) and after drying (37 °C) via dry combustion (LECO TruMac CN). The fresh char was collected from filter paper after all the free solution had drained, which took approximately 1–2 h. The volumetric moisture contents of the fresh chars were ca. 45% for the SS_A-C, 40% for SA and nearly 60% for BR and LG85.
To assess the amounts of elements released from the chars, a 24-h extraction was carried out with deionized water adjusted to pH 6 and 8 before equilibration with NaOH and H2SO4 similarly than for the N-containing solutions. The water extracts were analyzed for their pH (SevenCompact Duo Mettler Toledo, InLAb® Expert Pro-ISM), electrical conductivity (EC, SevenCompact Duo Mettler Toledo, InLAb® 731.ISM), total organic C (TOC-VCSH Shimadzu), NH4-N, NO3-N (Skalar San++System), and total concentrations of Al, Ca, Cu, Cd, Cr, Fe, K, Mg, Mn, P, Pb, S and Zn (ICP-OES, Perkin Elmer Optima 8300). In addition, the total N concentration of the chars was determined after water extraction when fresh using the Kjeldahl method and after drying (37 °C) via dry combustion. The (NH4)2SO4-equilibration-induced change in the char total N was calculated as the difference between N in the N-treated and water extracted chars so that the mass based N contents were converted to volume based concentrations using the char bulk densities.
The chars strongly buffered the suspension pH, wherefore the pH adjustment of the deionized water extract and the (NH4)2SO4-equilibration solutions to 6.0 or 8.0 before the treatment turned out to be negligible. Since the final pH values measured after the treatments were found exactly or nearly equal despite of the pH adjustment, the amounts of released elements in the water extracts and the sorption test results are presented as averages over the two initial solution pHs (six replicates for each treatment) and no pH adjustment was made in the second stage of N loading.
Nitrogen-Enriched Chars as N Fertilizers
The release of added NH4-N retained within the chars was assessed from three of the six chars. Based on the equilibration test results, one sewage sludge-derived char (SS_C), wood-based SA and manure-based BR were selected and the activated charcoal (LG85) and two SS chars were omitted from the study. Representative 2.5–4 L aliquots of the chars at a 0.6–2 mm particle size were loaded with an (NH4)2SO4 solution containing 5000 mg N L−1. The solution pH (5.4) was not adjusted. Due to technical challenges with the high volumes, a char to solution ratio of 1:5 was adopted, and the chars were treated in several batches each containing at least 0.2 L char. The suspensions were shaken at room temperature for 24 h, after which the chars were allowed to drain on filter paper for at most 1 h. Finally, the replicate batches of each char were combined and sampled for total N analysis. The moist chars were stored in an airtight container at + 4 °C until they were transferred to the pot experiment described below. The total N concentrations of the chars before and after the N loading were analyzed using the Kjeldahl method in three replicates per sample. In assessing the treatment-induced changes in the chars’ total N, the mass based values were converted to volume based concentrations using the bulk densities of the char.
The pot experiment was conducted with a sandy soil containing 4% clay (< 0.002 mm), 4% silt (0.002–0.02 mm), 58% fine sand (0.02–0.2 mm) and 34% sand (0.2–2 mm) in 8 L plastic pots (ø 22 cm) each containing 5.4 kg of soil (dry matter). The soil was limed to achieve a target pH of 6.5 and fertilized with granular superphosphate and nutrient solutions prepared of laboratory grade chemicals containing other nutrients than N. In total 1800 mg P, 4000 mg K, 9000 mg Ca, 300 mg Mg, 430 mg S, 20 mg Fe, Zn, and Mn, 10 mg Cu and 2 mg B and Mo was applied per pot for assuring that no other nutrient than N would limit growth [36]. The K application was split to 2000 mg pot−1 at establishment, 1000 mg pot−1 after the first harvest and 500 mg pot−1 after the following harvests to achieve a steady and adequate K supply.
The experimental treatments comprised five increasing mineral N (min-N, (Ca(NO3)2/NH4NO3; 25% NH4-N, 75% NO3-N) addition levels (0–1500 mg min-N pot−1) and three N enriched chars rationed to contain 1125 mg loaded N pot−1 as follows:
(1) min-N 0 (non-N-fertilized control),
(2) min-N 300 (300 mg N pot−1),
(3) min-N 750 (750 mg N pot−1),
(4) min-N 1125 (1125 mg N pot−1),
(5) min-N 1500 (1500 mg N pot−1),
(6) SS_C (1125 mg loaded N pot−1),
(7) SA (1125 mg loaded N pot−1),
(8) BR (1125 mg loaded N pot−1).
The char additions per pot were ca. 0.2 L in BR and SS_C and 0.4 L in SA (40.4, 125 and 76.4 g char DM, respectively). Each treatment was carried out in five replicates.
The chars and added nutrients were thoroughly mixed in the soil, after which water was added to moisten the soil to ca. 70% of its water holding capacity. Italian ryegrass (Lolium multiflorum var. Barmultra II) was sown at a rate of 360 mg seeds pot−1 to attain ca. 1 germinating seed per 4 cm2. Finally, the pots were arranged in a completely randomized design in an open-wall greenhouse. Deionized water was used for irrigation according to the needs of the plants. The grass leaf mass was harvested four times, 4 (Jul 11), 7 (Jul 28), 11 (Aug 28) and 17 (Oct 6) weeks after sowing (June 10) by cutting the grass to a height of 2 cm. The leaf mass was oven dried (+ 60 °C), weighed for the dry mass yield and analyzed for the total N using the Kjeldahl method.
The total N uptake in the aboveground leaf biomass was calculated by multiplying each leaf dry mass yield by its N concentration. An uptake response curve of N was fitted to the increasing mineral fertilizer-N additions and from this curve, the mineral-N equivalents of the N loaded in the chars were determined according to the N uptakes obtained with the N enriched chars. The division of the N uptake between the four subsequent harvests was observed using the apparent N recoveries determined as a proportion of the total N added that was recovered in the above ground plant biomass after subtracting the corresponding N content in non-N-fertilized plants.
Data Analysis
For the chars, four dependent variables (sorption of NH4-N, change in the total N at an equilibration ratio of 1:10 and 1:5 v/v, respectively, and the drying (+ 37 °C) induced change in the total N concentration) were analyzed using a linear mixed model (LMM) with the treatment (SS_A, SS_B, SS_C, SA and LG85), concentration level (0, 400, 1500 and 5000 mg N L−1), and their interaction as fixed effects. Correlated samples of concentration levels within each replicate were taken into account using an unstructured (UN) or a heterogeneous compound symmetry (CSH) covariance structure using the R-side random effect. For the change in the total N at the equilibration ratio of 1:5 v/v, a simplified model was used, where only three treatments (SS_C, SA and BR) were compared through the fixed effects. Unequal variances were allowed for the treatments in all models, based on a likelihood ratio test.
A similar two-way-interaction model for treatments was used for the N uptake of ryegrass, but the concentration level was replaced by the harvest (1 to 4). Correlated samples of harvests were taken into account using an UN covariance structure through the R-side random effect. For the total N uptake, a simplified model was used, where only four treatments (SS_C, SA, BR and min-N 1125) were compared through fixed effects. The assumption of equal variances was used for the treatments in both models.
The assumption of normality of the residuals was studied graphically from multiple residual plots and was found to be adequate for all models. The restricted maximum likelihood (REML) estimation method was used, and degrees of freedom were calculated using the Kenward–Roger method [37]. Tukey’s method [38] was used for pairwise comparisons of the means, with a significance level of α = 0.05. Treatments were compared within each concentration level or harvest to minimize the number of pairwise comparisons.
The analyses were performed using the GLIMMIX procedure of the SAS Enterprise Guide 7.15 (SAS Institute Inc., Cary, NC, USA).
Results and Discussion
Char Characteristics
Feedstock sources are known to have a major influence on biochar properties [e.g. 8, 39–40], and for this reason chars derived from contrasting materials were chosen for testing (Table 1). The general differences between wood- (SA), sewage sludge- (SS_A-C) and manure-based (BR) chars of the current study produced at rather low temperature (450–550 °C) through slow pyrolysis agreed with previous findings. The wood-based chars tend to exhibit a higher specific surface area and total C content but a lower CEC and total and available element concentrations than manure- and biosolids-based chars [12]. The elemental composition of the water extracts showed the activated charcoal (LG85) to be very poor in available elements, whereas the manure-based BR released K, P and S. Broiler manure is one of the nutrient richest manures and in our previous study [9] the peat bedded broiler manure feedstock was found to contain 24.9, 14.9 and 5.8 g K, P and S per kg dry mass (dm), respectively. The wood-based SA released some K, and the sewage-sludge-based chars mainly released Ca and S but also some N (Table 2, Sect. 2.2). According to the extensive review of Ippolito et al. [12] wood-based chars contain relatively high amounts of K (on average nearly 20 g total K kg−1 dm), whereas in biosolids derived chars, the mean total concentrations of Ca (ca. 50 g kg−1 dm), S (ca. 1 g kg−1 dm) and N (ca. 2.5 g kg−1 dm) are the highest among feedstock sources. However, predicting plant availability of nutrients based on their total concentrations is somewhat complicated and dependent on the feedstock material and nutrient element [12]. Considering the inherent fertilizer value of the current chars based on their water-extractable element concentrations, the BR would be most promising by providing ca. 35 kg K, 1 kg P and 1.5 kg S per dm ton in easily plant available form, but also the easily available K content of the SA (ca. 3.5 kg t−1 dm) is worth mentioning.
All the studied chars turned the water extracts alkaline, but the BR clearly had the highest pH (Table 2), which is typical for chars derived from high-ash animal manures [e.g. 8, 41]. The pH of the sewage sludge-derived chars has been shown to increase from an acidic range to become highly alkaline with an increase in the production temperature [42, 43]. Moreover, sewage sludge-based chars contain high amounts of metal rich ash [44, 45] and are relatively heavy (Table 1).
The specific surface areas in Table 1 showing orders of magnitude differences between the char types, reflect the nano-porosity of the materials, which is less relevant regarding the present application than micrometer-scale pores allowing rapid solution flow [46]. Previously, Rasa et al. [11] found willow (Salix) biochar to retain the initial internal structure of the fresh wood so that the vascular tissues formed a total char porosity of 0.6. In broiler manure biochar, which is equivalent to the current BR, Keskinen et al. [9] observed a more versatile pore structure in comparison to the willow char, although the total porosity was likewise around 0.6. In the current sewage sludge-based chars, Turunen et al. [32] recorded a pore space consisting of small individual pores and crevices accompanied by large spherical cavities. The total porosity values of the SS chars remained slightly below 0.2, and they had no effect on soil or growing media water retention properties.
Nitrogen Enrichment
The equilibration solution pH values measured after the treatment decreased with an increase in the solution (NH4)2SO4 concentration so that average ranges of 7.5–7.0 in the sewage sludge- based chars (SS_A,B and C), 8.0–7.5 in SA, 9.2–8.2 in BR and 8.6–7.9 in LG85 were obtained. Kizito et al. [23], Fan et al. [28] and Tang et al. [33] reported NH4-N adsorption onto chars derived from rice husks, wood, bamboo, and sewage sludge to increase with an increase in the pH value from around 3 up to 7 due to deprotonation of surface functional groups, but beyond pH 7–8 begin to decrease, which was possibly due to the conversion of NH4+ to poorly adsorbing ammonia (NH3). In the current study, adjusting the pH to around 7 during the equilibration might thus have slightly increased the adsorption for the most alkaline chars (SA, BR, LG85).
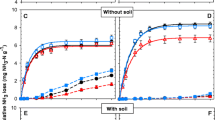
At 400 mg NH4-N L−1 equilibration solution concentration, BR and LG85 exhibited the highest N adsorption capacity of nearly 1 g NH4-N L−1 of char (Fig. 1), which corresponded to ca. 20% removal of the total N in the equilibration solution. In SA, SS_B and SS_C, only ca. 0.2 g NH4-N was adsorbed per L of char and the N removal efficiency was thus merely roughly 5%. In SS_A, no N sorption was detected at the 400 mg NH4-N L−1 equilibration concentration level but rather the char released some N.
Net adsorption of NH4-N (mg L−1 char) at equilibrium solution concentrations of 400, 1500 and 5000 mg NH4-N L−1 as (NH4)2SO4. The results are mean estimates with a ± 95% confidence interval. The values are slightly displaced horizontally for clarity. Statistically significant differences between chars within equilibration levels are denoted with different letters (p < 0.05)
At higher equilibration concentrations (1500 and 5000 mg NH4-N L−1), the relative changes in the equilibration solution NH4-N remained marginal, which led to a rather high deviation between the replicates and thus to loss of significant differences between the char adsorption capacities (Fig. 1). Though the mean values of the adsorbed N tended to increase with an increasing solution N concentration, a significant difference in the amount of adsorbed N between the N levels was found only for the SS_A between 400 and 5000 mg N L−1 solution concentrations (p = 0.01, SE = 0.44). Overall, the maximum N adsorption capacity of the studied chars seemed to be limited to around 0.5–2 g N L−1 char. On a mass basis, the maximum adsorbed values corresponded to ca. 1–2 mg N g−1 for the SS chars, 3.5 mg g−1 for LG85 and 7.5–8 mg g−1 for SA and BR.
In comparison to previous studies covering various feedstocks, process conditions, equilibration solution NH4-N concentrations, char:solution ratios, pH levels and particle sizes, the current range of maximum adsorption seems typical. The reported values mainly range from zero adsorption or a slight N release up to around 10 mg NH4-N retention per g char, with the values centering below 5 mg g−1 [e.g. 20, 24, 25, 29, 30, 33, 47–49]. Only in single studies have the adsorption of several dozens [22, 23] or over 100 mg NH4-N g−1 char [26] been reported. Multiple processes have been found to be involved in the retention of NH4-N in char and the main mechanism probably depends on the char properties, e.g. quantity and quality of surface functional groups able to bind NH4+ via electrostatic interactions and morphological characteristics governing physical sorption [22,23,24,25, 50]. In chars rich in ash, such as the SS_A-C and BR in the current study, the surface precipitation of e.g. struvite (MgNH4PO4) may also play a role in the N retention [28]. Identifying the specific sorption mechanisms was beyond the scope of this study.
The N concentrations of the chars, measured via the Kjeldahl digestion of the moist char material, in addition to the adsorbed N discussed above, included N carried in the solution retained within the char pores and between the char particles after free gravity drainage. At 400 mg NH4-N L−1 equilibration level, changes in the char total N concentration from the average values obtained after water extraction roughly agreed with the adsorption/desorption values (Fig. 1, Table 3). In exception, the LG85 exhibited a clearly lower increase in char’s total N than the decrease recorded in the equilibration solution N, which may be related to NH3 evaporation (see below). In all chars, the amount of N retained significantly increased with an increase in the equilibration solution N concentration (p < 0.05, SE = 0.25–0.29), and at higher solution N concentrations, the increases in the char’s total N exceeded the decreases in the equilibration solution N. Considering the porosity values determined for the present chars in previous studies (0.6 for SA and BR, and ca. 0.2 for the SS chars; [9, 11, 32], the SS_chars could be expected to be able to hold less solution than the more porous chars. However, the retention of N solution between the char particles was also possible, but dependent on the particle size distribution within the applied range (0.6–2 mm), which was not specified. Furthermore, the spatial organization of the pore space and possible hydrophobic characteristics of the chars may also have controlled the absorption of the N solution. In the end, a comparison of the moisture contents of the fresh chars revealed that the SA chars held less solution (ca. 40% v/v) than the SS (ca. 45%) and BR and LG85 chars (ca. 60%).
In general, the differences in the total N retention capacity between the chars were small when considering the true fertilizer value of the products, though the BR char tended to retain the highest amounts of added N (Table 3). It is noteworthy that the activated charcoal (LG85) was equal in performance to the slow pyrolyzed chars derived from low value feedstocks. At a 1:10 equilibration ratio, in total roughly 2–4 g NH4-N L−1 char could be loaded using a 5000 mg NH4-N L−1 solution. Narrowing the char:solution ratio to 1:5 and increasing the batch size resulted in a further increase in the char total N. This effect was most pronounced for the SS_C char and may be explained by the increased ionic strength which enhanced the NH4-N adsorption [28].
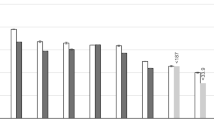
The differences between the total N concentrations of the chars when fresh and after drying at 37 °C revealed the BR and LG85 chars to be susceptible to gaseous loss of the loaded N (Fig. 2). The amount of N lost from these chars corresponded at the most to 40% of the amount added during equilibration. This finding can be explained by the pH values of the chars and increased temperature during the treatment. The proportion of volatile NH3 to aqueous NH4+ increases sharply with an increase in pH in the alkaline range [e.g. 51]. In addition, the proportion of NH3 increases with an increase in temperature [35, 51]. Consequently, in the highly alkaline chars (BR and LG85 exhibiting pH of around or above 8 in the equilibration solution) the free NH4+ not directly attached to the char could easily be lost. Furthermore, in these chars, the moisture content when fresh was the highest, suggesting presence of free NH4-N containing solution retained within the char pores (see Sect. 2.2). In the SA and SS chars, in which no significant loss of N was observed, the pH was lower though also alkaline. Besides the pH, the lack of NH3 volatilization may be explained by differences in the N retention mechanisms (entrapment, precipitation, adsorption), which could not be differentiated in the current study.
Nitrogen Release
Over the four consecutive ryegrass harvests, the total N uptake per pot decreased in the order of min-N > SA > BR > SS_C as the N source (Fig. 3). According to the N uptake response curve, 89 ± 4, 62 ± 5 and 43 ± 4% of the enriched N was equivalent to the min-N in the SA, BR, and SS_C chars, respectively.
The ryegrass N uptake response curve to mineral fertilizer-N calculated over four consecutive harvests in a pot experiment. The mineral-N equivalents of N enriched chars (application rate 1125 mg N pot−1) are determined from the response curve according to the N uptakes obtained. The values are mean estimates with ± 95% confidence intervals. Statistically significant differences among the uptake values are denoted with different letters (p < 0.05)
The total apparent N recovery over the four consecutive harvests was 75, 67, 47 and 34% of the added N in the min-N 1125, SA, BR and SS_C treatments, respectively (SE = 1.5). From all the N sources, the highest N recovery occurred during the first hay growth and decreased thereafter with a gradually depleting supply (Fig. 4). In the first cut, significantly less N was acquired from the SS_C than from the SA and BR chars or mineral fertilizer, whereas in the second cut the mineral fertilizer addition supplied more N than the chars, among which the SA char could be discerned as the best N source. In the third cut, the N recoveries for the min-N and SA treatments were equal and somewhat higher than from the BR and SS_C treatments. In the final fourth cut, no significant differences in the N recovery of the different treatments occurred.
The apparent N recovery values of the total N added in the aboveground ryegrass leaf biomass over four consecutive harvests. The values are mean estimates with + 95% confidence intervals. Statistically significant differences between the N sources within cuts are denoted with different letters (p < 0.05)
The results demonstrated that the N loaded in the SA char was easily released for plant uptake, whereas for the BR and especially SS_C chars a considerable part of the N enrichment was not available to plants. In light of the findings on the gaseous N loss (Fig. 2), it can be assumed that volatilization explained part of the low N availability in the BR char. However, both in the SS_C and BR chars, retention of N may have occurred via surface precipitation and complex formation making the N scarcely available.
In previous studies, release of N has mainly been examined through salt extraction of the loaded chars [22, 26, 30, 50]. In the majority of cases, very small amounts (< 1%–ca. 5%) of the retained N was released but high releasing ratios of 40–70% have also been observed [30, 50]. Kocatürk-Schumacher et al. [31] conducted a pot experiment with ryegrass to examine the plant availability of N enriched in wood-based biochar and recorded an apparent N recovery of merely roughly 10%, which was attributed to high N retention. Clearly major differences in the strength of N retention occur between chars and both the sorption and release capacities need to be assessed while estimating the feasibility of different chars as N carriers.
Due to the observed strong retention of the added N, N enriched chars have been proposed as slow-releasing fertilizers in several studies [e.g. 19, 28, 50]. However, the exhaustive four consecutive harvests collected in the current study showed no slow-release characteristics for any of the chars (Fig. 4). On the contrary, the available N ran out fastest in the BR and SS_C chars with the lowest total N supply. Therefore, any possible slow release of the char recalcitrant N cannot be considered to hold any true agronomic fertilizer value.
Practical Considerations on the Production and Fertilizer Value of N Enriched Chars
In the current study, differences in the capacity of the chars derived from contrasting feedstocks (sewage sludge, wood, manure, coal) to retain added N were minor, but significant differences were observed in their ability to release the loaded N. Previous studies indicate major differences in both characteristics and evidently the processes of sorption and release are governed by multiple factors and no single feedstock type or process condition can yet be generally identified as superior.
The current, rather typical rate of maximum N loading, limited to roughly 5 g N l−1 char, is small from an agricultural fertilizer point of view. In crop production, N fertilization levels of around 100 kg N ha−1 are typically used per crop. To supply this amount of loaded N using the studied chars, addition levels of 25–50 m3 or on a mass basis (dry weight) ca. 30 t of SS char, 10 t of SA, 5 t of BR and 15 t of LG85 would be needed. For chars exhibiting restricted N release, as shown for the BR and SS chars in the current study, the amounts would need to be roughly doubled to reach desired plant-available levels. In the case of chars produced from feedstocks rich in nutrients or metals (BR, SS), the application rates are limited due to these loads. Keskinen et al. [9] reported that an addition of merely 1 t BR char ha−1 would supply 30–35 kg total P. In the SS-based chars the amount of P may exceed that of chars derived from animal manure and the high heavy metal contents, though mainly non-bioavailable, need to be accounted for [52]. These chars might thus be feasible N carriers only under low-N demand, namely in landscaping or long-lasting growing media dedicated to decorative perennials in urban areas. However, an observable slow release of N would be desirable in these applications.
Of the studied chars, only the woody SA, with low inherent nutrient and metal contents which would not restrict high application dosages could be used to supply agronomically sufficient amounts of N. The high addition of recalcitrant carbon introduced with a high SA application would also be beneficial considering the C sequestration, but obviously this kind of product would not be appropriate for frequent use. Although the N retention capacity of the SA char was not the highest attained, the N captured was readily plant available but sufficiently attached to avoid gaseous losses if the moist material would be dried to facilitate transportation and storage. However, the effects of drying on the availability of added N is not known. Due to the easy release of N from the moist char, the SA char could also provide N only for one growing season or for a single crop.
A major motivation for the char N enrichment, besides increasing the value of the char as a bio-based fertilizer, is the anticipated double benefit arising from the first step of wastewater purification in the cascade use of chars. Typically, the NH4-N removal percentage is high, even up to 90% in solutions with low initial NH4-N concentration, but the removal percentage tends to decrease to < 20% as the solution NH4-N concentration increases, although the total amount of N adsorbed increases with an increase in the solution NH4-N concentration [23, 30, 33]. In the current study, the maximum removal of around 20% of the N in a solution containing 400 mg N L−1 was attained using a 1:10 char to solution ratio. Considering industrial wastewater, this concentration is not exceptionally high. Due to the limited adsorption capacity of the studied chars, the available sorption sites are saturated quickly, and large char volumes would be needed for efficient enough water purification [47]. From a practical point of view, exceptional chars with a multiple adsorption capacity in comparison to the chars of this study would probably be needed for the economic production of bio-based fertilizer chars enriched with NH4-N.
Conclusions
Differences in the N retention capacity of chars derived from sewage sludge, Salix wood, broiler manure, and coal were small in relation to agronomic efficiency. The amounts of total NH4-N retained within moist chars, including both directly adsorbed N and that present as free ions in solution retained within and between char particles increased with an increase in the N solution concentration, reaching 2–4 g L−1 char at a solution concentration of 5000 mg NH4-N L−1. The corresponding direct adsorption capacity was limited to 0.5–2 g N L−1 char. The N release differed significantly between the studied chars. In four consecutive harvests, the apparent N recovery was 67, 47 and 34% for the added N in SA, BR, and SS_C chars, while the mineral N equivalences were 89, 62 and 43%, respectively. No slow-release of N was observed for any of the chars. Highly alkaline chars probably carrying free NH4+ ions were prone to gaseous losses of N upon drying. From an agronomic point of view, the amounts of N retained within the chars were small. Accounting for the inherent nutrient and metal contents of the chars dependent on the feedstock material, only the SA char could be applied in the volumes needed to supply sufficient amounts of N for crop production, but such high volumes are inappropriate for frequent use. As true fertilizers, the current N enriched chars can merely complement the principal N sources. Chars with a higher N retention capacity would be needed for an efficient cascade from water purification to fertilizer use.
Data Availability
The data is available from the authors upon reasonable request.
References
Laird, D.A., Brown, R.C., Amonette, J.E., Lehmann, J.: Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuel. Bioprod. Biorefin. 3, 547–562 (2009)
Libra, J.A., Ro, K.S., Kammann, C., Funke, A., Berge, N.D., Neubauer, Y., Titirici, M.-M., Fühner, C., Bens, O., Kern, J., Emmerichet, K.-H.: Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2, 71–106 (2011)
Paneque, M.D., la Rosa, J.M., Kern, J., Reza, M.T., Knicker, H.: Hydrothermal carbonization and pyrolysis of sewage sludges: what happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 128, 314–323 (2017)
Sohi, S.P., Krull, E., Lopez-Capel, E., Bol, R.: A review of biochar and its use and function in soil. In: Advances in Agronomy, pp. 47–82. Elsevier, Amsterdam (2010)
Zielińska, A., Oleszczuk, P.: Effect of pyrolysis temperatures on freely dissolved polycyclic aromatic hydrocarbon (PAH) concentrations in sewage sludge-derived biochars. Chemosphere 153, 68–74 (2016)
Atkinson, C.J., Fitzgerald, J.D., Hipps, N.A.: Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337, 1–18 (2010)
Jeffery, S., Verheijena, F.G.A., van der Velde, M., Bastos, A.C.: A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 144, 175–187 (2011)
Enders, A., Hanley, K., Whitman, T., Joseph, S., Lehmann, J.: Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 114, 644–653 (2012)
Keskinen, R., Hyväluoma, J., Sohlo, L., Help, H., Rasa, K.: Fertilizer and soil conditioner value of broiler manure biochars. Biochar 1, 259–270 (2019)
Novak, J.M., Cantrell, K.B., Watts, D.W.: Compositional and thermal evaluation of lignocellulosic and poultry litter chars via high and low temperature pyrolysis. Bioenergy Res. 6, 114–130 (2013)
Rasa, K., Heikkinen, J., Hannula, M., Arstila, K., Kulju, S., Hyväluoma, J.: How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenergy 119, 346–353 (2018)
Ippolito, J.A., Cui, L., Kammann, C., Wrage-Mönning, N., Estavillo, J.M., Fuertes-Mendizabal, T., Cayela, M.L., Sigua, G., Novak, J., Spokas, K., Borchard, N.: Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2, 421–438 (2020)
Leng, L., Xu, S., Liu, R., Yu, T., Zhuo, X., Leng, S., Xiong, Q., Huang, H.: Nitrogen containing functional groups of biochar: an overview. Bioresour. Technol. 298, 122286 (2020)
Pels, J.R., Kapteijn, F., Moulijn, J.A., Zhu, Q., Thomas, K.M.: Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 33, 1641–1653 (1995)
Ren, Q., Zhao, C.: NOx and N2O precursors from biomass pyrolysis: nitrogen transformation from amino acid. Environ. Sci. Technol. 46, 4236–4240 (2012)
Ro, K.S., Cantrell, K.B., Hunt, P.G.: High-temperature pyrolysis of blended animal manures for producing renewable energy and value-added biochar. Ind. Eng. Chem. Res. 49, 10125–10131 (2010)
Wang, T., Camps Arbestain, M., Hedley, M., Bishop, P.: Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 51, 42–54 (2012)
Song, W., Guo, M.: Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 94, 138–145 (2012)
Clough, T.J., Condron, L.M., Kammann, C., Müller, C.: A review of biochar and soil nitrogen dynamics. Agronomy 3, 275–293 (2013)
Gai, X., Wang, H., Liu, J., Zhai, L., Liu, S., Ren, T., Liu, H.: Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 9, e113888 (2014)
Hale, S.E., Alling, V., Martinsen, V., Mulder, J., Breedveld, G.D., Cornelissen, G.: The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 91, 1612–1619 (2013)
Jassal, R.S., Johnson, M.S., Molodovskaya, M., Black, T.A., Jollymore, A., Sveinson, K.: Nitrogen enrichment potential of biochar in relation to pyrolysis temperature and feedstock quality. J. Environ. Manag. 152, 140–144 (2015)
Kizito, S., Wu, S., Kirui, W.K., Lei, M., Lu, Q., Bah, H., Dong, R.: Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 505, 102–112 (2015)
Li, S., Barreto, V., Li, R., Chen, G., Hsieh, Y.P.: Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 133, 136–146 (2018)
Yin, Q., Liu, M., Ren, H.: Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 249, 109410 (2019)
Takaya, C.A., Fletcher, L.A., Singh, S., Anyikude, K.U., Ross, A.B.: Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145, 518–527 (2016)
Tian, J., Miller, V., Chiu, P.C., Maresca, J.A., Guo, M., Imhoff, P.T.: Nutrient release and ammonium sorption by poultry litter and wood biochars in stormwater treatment. Sci. Total Environ. 553, 596–606 (2016)
Fan, R., Chen, C., Lin, J., Tzeng, J., Huang, C., Dong, C., Huang, C.P.: Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 272, 465–472 (2019)
Zhang, J., Wang, Q.: Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J. Clean. Prod. 112, 3927–3934 (2016)
Sarkhot, D.V., Ghezzehei, T.A., Berhe, A.A.: Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 42, 1545–1554 (2013)
Kocatürk-Schumacher, N.P., Zwart, K., Bruun, S., Jensen, L.S., Sørensen, H., Brussaard, L.: Recovery of nutrients from the liquid fraction of digestate: use of enriched zeolite and biochar as nitrogen fertilizers. J. Plant Nutr. Soil Sci. 182, 187–195 (2019)
Turunen, M., Hyväluoma, J., Keskinen, R., Kaseva, J., Nikama, J., Reunamo, A., Rasa, K.: Pore structure of wastewater sludge chars and their water retention impacts in different soils. Biosyst. Eng. 206, 6–18 (2021)
Tang, Y., Alam, M.S., Konhauser, K.O., Alessi, D.S., Xu, S., Tian, W., Liu, Y.: Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 209, 927–936 (2019)
Fangueiro, D., Hjorth, M., Gioelli, F.: Acidification of animal slurry—a review. J. Environ. Manag. 149, 46–56 (2015)
Martinelle, K., Häggström, L.: On the dissociation constant of ammonium: effects of using an incorrect pKa in calculations of the ammonia concentration in animal cell cultures. Biotechnol. Tech. 11, 549–551 (1997)
Keskinen, R., Suojala-Ahlfors, T., Sarvi, M., Hagner, M., Kaseva, J., Salo, T., Uusitalo, R., Rasa, K.: Granulated broiler manure based organic fertilizers as sources of plant available nitrogen. Environ. Technol. Innov. 18, 100734 (2020)
Kenward, M.G., Roger, J.H.: An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stat. Data Anal. 53, 2583–2595 (2009)
Westfall, P., Tobias, R.D., Wolfinger, R.D.: Multiple comparisons and multiple tests using SAS. SAS Publishing, USA (2011)
Novak, J.M., Cantrell, K.B., Watts, D.W., Busscher, W.J., Johnson, M.G.: Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 14, 330–343 (2014)
Zhao, L., Cao, X., Mašek, O., Zimmerman, A.: Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 256–257, 1–9 (2013)
Cantrell, K.B., Hunt, P.G., Uchimiya, M., Novak, J.M., Ro, K.S.: Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 107, 419–428 (2012)
Hossain, M.K., Strezov, V., Chan, K.Y., Ziolkowski, A., Nelson, P.F.: Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 92, 223–228 (2011)
Yuan, H., Lu, T., Huang, H., Zhao, D., Kobayashi, N., Chen, Y.: Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 112, 284–289 (2015)
Agrafioti, E., Bouras, G., Kalderis, D., Diamadopoulos, E.: Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 101, 72–78 (2013)
Zhang, J., Lü, F., Zhang, H., Shao, L., Chen, D., He, P.: Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci. Rep. 5, 9406 (2015)
Hyväluoma, J., Hannula, M., Arstila, K., Wang, H., Kulju, S., Rasa, K.: Effects of pyrolysis temperature on the hydrologically relevant porosity of willow biochar. J. Anal. Appl. Pyrolysis 134, 446–453 (2018)
Beckinghausen, A., Reynders, J., Merckel, R., Wu, Y.W., Marais, H., Schwede, S.: Post-pyrolysis treatments of biochars from sewage sludge and A. mearnsii for ammonia (NH4-n) recovery. Appl. Energy 271, 115212 (2020)
Sarkhot, D.V., Berhe, A.A., Ghezzehei, T.A.: Impact of biochar enriched with dairy manure effluent an carbon and nitrogen dynamics. J. Environ. Qual. 41, 1107–1114 (2012)
Zeng, Z., Zhang, S., Li, T., Zhao, F., He, Z., Zhao, H., Yang, X., Wang, H., Zhao, J., Rafiq, M.T.: Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J. Zhejiang Univ. Sci. B 14, 1152–1161 (2013)
Cai, Y., Qi, H., Liu, Y., He, X.: Sorption/desorption behavior and mechanism of NH4+ by biochar as a nitrogen fertilizer sustained-release material. J. Agric. Food Chem. 64, 4958–4964 (2016)
Hartung, J., Phillips, V.R.: Control of gaseous emissions from livestock buildings and manure stores. J. Agric. Eng. Res. 57, 173–189 (1994)
Liu, T., Liu, B., Zhang, W.: Nutrients and heavy metals in biochar produced by sewage sludge pyrolysis: its application in soil amendment. Pol. J. Environ. Stud. 23, 271–275 (2014)
Acknowledgements
We wish to acknowledge Luke’s technical staff in Jokioinen for the excellent contribution. The company Mayt Oy is acknowledged for providing a pilot-scale pyrolysis device for the production of willow biochar. The work received funding from the Ministry of Education and Culture, Finland, through the Bioproduct and Clean Bioeconomy—an RDI FlagShip at Xamk project and from the Ministry of the Environment of Finland from the program to promote the recycling of nutrients and improve the status of the Archipelago Sea through the Lietehiili project.
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE). This study was funded by the Ministry of Education and Culture, Finland, through the Bioproduct and Clean Bioeconomy—an RDI FlagShip at Xamk project and the Ministry of the Environment of Finland from the program to promote the recycling of nutrients and improve the status of the Archipelago Sea through the Lietehiili project.
Author information
Authors and Affiliations
Contributions
Conceptualization: RK, JN, KR. Methodology: RK, KR. Formal analysis and investigation: JK. Writing—original draft preparation: RK, JK. Writing—review and editing: RK, JN, JK, KR. Funding aquisition: KR.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to Participate
All authors are consent to participate in the study and all persons entitled to authorship have been so named.
Consent for Publication
The submission of this work for publication has been approved by all authors and institutions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keskinen, R., Nikama, J., Kaseva, J. et al. Feasibility of Nitrogen-Enriched Chars as Circular Fertilizers. Waste Biomass Valor 12, 6823–6833 (2021). https://doi.org/10.1007/s12649-021-01471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01471-5