Abstract
Background and aims
Salix integra has a high tolerance to lead (Pb) stress, and has been applied in the phytostabilization of Pb-Zn mine tailings in East China. Mechanisms for Pb sequestration and detoxification were poorly understood in Salix plants. The present study aimed to elucidate where Pb was localized and how Pb was combined in roots of S. integra for a better understanding of Pb tolerance strategies.
Methods
Histochemical methods combined with synchrotron-based X-ray fluorescence microscopy, transmission electron microscopy and fourier transform infrared spectroscopy were used to explore the distribution and chemical forms of Pb at cellular and subcellular levels in S. integra.
Results
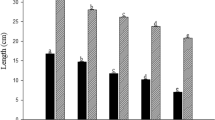
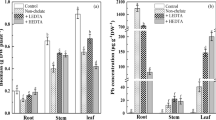
The accumulation of Pb displayed a fast linear uptake in roots and stems in the short-term period, whereas a slower Freundlich-like uptake in leaves. Micro-XRF and histochemical staining revealed that the absorbed Pb in roots was mostly restricted in the cortex, and partly translocated in the stele. At the subcellular level, most Pb in roots was localized in the cell walls and trophoplast fraction, and the free -OH and pectin C—O/C—O—S groups may be involved in the cell wall sequestration. It was also found that the majority of Pb in roots existed as phosphate and oxalate.
Conclusions
S. integra has a high uptake potential of accumulating Pb in their roots. The absorbed Pb was primarily localized in the cell walls and combined with -OH or pectin groups, and precipitation as phosphate or oxalate may be responsible for the detoxification in root cells.





Similar content being viewed by others
References
Arias JA, Peralta-Videa JR, Ellzey JT, Ren M, Viveros MN, Gardea-Torresdey JL (2010) Effects of Glomus deserticola inoculation on Prosopis:enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ Exp Bot 68(2):139–148
Cao Y, Ma C, Zhang J, Wang S, White JC, Chen G, Xing B (2019) Accumulation and spatial distribution of copper and nutrients in willow as affected by soil flooding: a synchrotron-based X-ray fluorescence study. Environ Pollut 246:980–989
Chen GC, Liu YQ, Wang RM, Zhang JF, Owens G (2013) Cadmium adsorption by willow root: the role of cell walls and their subfractions. Environ Sci Pollut Res 20:5665–5672
Chudzik B, Szczuka E, Leszczuk A, Strubińska J (2018) Modification of pectin distribution in sunflower (Helianthus annuus L.) roots in response to lead exposure. Environ Exp Bot 155:251–259
Cotter-Howells J, Champness P, Charnock J (1999) Mineralogy of Pb-P grains in the roots of Agrostis capillaris L. by ATEM and EXAFS. Mineral Mag 63:777–789
Dos Santos Utmazian MN, Wieshammer G, Vega R, Wenzel WW (2007) Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut 148:155–165
Fu X, Dou C, Chen Y, Chen X, Shi J, Yu M, Xu J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186(1):103–107
Ginn BR, Szymanowski JS, Fein JB (2008) Metal and proton binding onto the roots of fescue rubra. Chem Geol 253(3–4):130–135
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Applied Ecology & Environmental Research 3(1):1–18
Gupta DK, Huang HG, Corpas FJ (2013) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161
Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouhe M (2010) The detoxification of lead in Sedum alfredii H. is not related to but the glutathione. J Hazard Mater 177:437–444
Harada E, Hokura A, Takada S, Baba K, Terada Y, Nakai I, Yazaki K (2010) Characterization of cadmium accumulation in willow as a woody metal accumulator using synchrotron radiation-based X-ray microanalyses. Plant & cell physiology 51:848–853
Huang L, Zhang H, Song Y, Yang Y, Chen H, Tang M (2017) Subcellular compartmentalization and chemical forms of Lead participate in Lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front Plant Sci 8:517
Inoue H, Fukuoka D, Tatai Y, Kamachi H, Hayatsu M, Ono M, Suzuki S (2013) Properties of lead deposits in cell walls of radish (Raphanus sativus) roots. J Plant Res 126:51–61
Islam E, Yang X, Li TQ, Liu D, Jin XF, Meng FH (2007) Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 147(3):806–816
Kopittke PM, CJF A, Blamey PC, Auchterlonie GJ, Guo YN, Menzies NW (2008) Localization and chemical speciation of Pb in roots of signal grass (Brachiaria decumbens) and Rhodes grass (Chloris gayana). Environ Sci Technol 42(12):4595–4599
Krzeslowska M, Rabda I, Aneta B, Lewandowski M, Wony A (2016) Pectinous cell wall thickenings formation - a common defense strategy of plants to cope with Pb. Environ Pollut 214:354–361
Ksiazek M, Wozny A (1990) Lead movement in poplar adventitious roots. Biol Plant 32(1):54–57
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29(5):1232–1238
Lane SD, Martin ES (1977) A histochemical investigation of lead uptake in Raphanus sativus. New Phytol 79(2):281–286
Largo-Gosens A, Hernandez-Altamirano M, Garcia-Calvo L, Alonso-Simon A, Alvarez J, Acebes J (2014) Fourier transform mid infrared spectroscopy applications for monitoring the structural plasticity of plant cell walls. Front Plant Sci 5:303
Li M, Cheng XH, Guo HX (2013) Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad 76:81–85
Liu YQ, Chen GC, Zhang JF, Shi X, Wang RM (2011) Uptake of cadmium from hydroponic solutions by willows (Salix spp.) seedlings. Afr J Biotechnol 10(72):16209–16218
Malecka A, Piechalak A, Tomaszewska B (2009) Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: the whole roots level. Acta Physiol Plant 31:1053–1063
Małecka A, Piechalak A, Morkunas I, Tomaszewska B (2008) Accumulation of lead in root cells of Pisum sativum. Acta Physiol Plant 30:629–637
Meyers DER, Auchterlonie GJ, Webb RI, Wood B (2008) Uptake and localisation of lead in the root system of Brassica juncea. Environ Pollut 153(2):323–332
Marmiroli M, Pietrini F, Maestri E, Zacchini M, Marmiroli N, Massacci A (2011) Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiol 31(12):1319–1334
Mleczek M, Gasecka M, Waliszewska B, Magdziak Z, Szostek M, Rutkowski P, Kaniuczak J, Zborowska M, Budzynska S, Mleczek P, Niedzielski P (2018) Salix viminalis L. - a highly effective plant in phytoextraction of elements. Chemosphere 212:67–78
Mun HW, Hoe AL, Koo LD (2008) Assessment of Pb uptake, translocation and immobilization in kenaf (Hibiscus cannabinus L.) for phytoremediation of sand tailings. J Environ 20(11):1341–1347
Parrotta L, Guerriero G, Sergeant K, Cai G andHausman JF (2015) Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Frontiers in Plant Science 6:133
Pulford ID, Watson C (2003) Phytoremediation of heavy metal-contaminated land by trees—a review. Environ Int 29(4):529–540
Polec-Pawlak K, Ruzik R, Lipiec E, Ciurzynska M, Gawronska H (2007) Investigation of Pb (II) binding to pectin in Arabidopsis thaliana. J Anal At Spectrom 22:968–972
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contam Toxicol 213:113–136
Pourrut B, Shahid M, Douay F, Dumat C, Pinelli E (2013) Molecular mechanisms involved in Lead uptake, toxicity and detoxification in higher plants. In: Gupta D, Corpas F, Palma J (eds) Heavy metal stress in plants. Springer
Rytwo G, Zakai R, Wicklein B (2015) The use of ATR-FTIR spectroscopy for quantification of absorbed compounds. Journal of Spectroscopy 2015:8
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Seregin IV, Ivanov VB (1997) Histochemical investigation of cadmium and lead distribution in plants. Russ J Plant Physiol 44(6):791–796
Seregin IV, Shpigun LK, Ivanov VB (2004) Distribution and toxic effects of cadmium and Lead on maize roots. Russ J Plant Physiol 51(4):525–533
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 219:1–12
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17(1):35–52
Sharma SS, Dietz KJ, Mimura T (2016) Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell and Environment 39(5):1112–1126
Shi X, Wang S, Sun H, Chen Y, Wang D, Pan H, Zou Y, Liu J, Zheng L, Zhao X, Jiang Z (2017) Comparative of Quercus spp. and Salix spp. for phytoremediation of Pb/Zn mine tailings. Environ Sci Pollut Res Int 24:3400–3411
Shi X, Wang SF, Wang DX, Sun HJ, Chen YT, Liu JF, Jiang ZP (2019) Woody species Rhus chinensis mill. Seedlings tolerance to Pb: physiological and biochemical response. J Environ Sci 78:63–73
Solé VA, Papillon E, Cotte M, Walter P, Susini J (2007) A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B 62:63–68
Sun J, Luo L (2018) Subcellular distribution and chemical forms of Pb in corn: strategies underlying tolerance in Pb stress. J Agric Food Chem 66(26):6675–6682
Tlustos P, Pavlíková D, Száková J, Balik J (2006) Plant accumulation capacity for potentially toxic elements. In: Morel JK (eds.), phytoremediation of metal-contaminated soils. Springer Netherlands 2006:53–84
Tripathi DK, Singh VP, Prasad SM, Dubey NK, Chauhan DK, Rai AK (2016) LIB spectroscopic and biochemical analysis to characterize lead toxicity alleviative nature of silicon in wheat (Triticum aestivum L.) seedlings. J Photochem Photobiol B 154:89–98
Tung G, Temple PJ (1996) Uptake and localization of lead in corn (Zea mays L.) seedlings, a study by histochemical and electron microscopy. Sci Total Environ 188:71–85
Vandecasteele B, Quataert P, De Vos B, Tack FG, Muys B (2004) Foliar concentrations of volunteer willows growing on polluted sediment-derived sites versus sites with baseline contamination levels. J Environ Monitoring 6:313–332
Vollenweider P, Cosio C, Günthardt-Goerg MS, Keller C (2006) Localization and effects of cadmium in leaves of a cadmium-tolerant willow (Salix viminalis L.). Environ Exp Bot 58(1–3):25–40
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) gaud. Environ Exp Bot 62:389–395
Wang SF, Shi X, Sun HJ, Chen YT, Pan HW, Yang XE, Rafiq T (2014) Variations in metal tolerance and accumulation in three hydroponically cultivated zarieties of Salix integra treated with lead. PLoS ONE:e108568
Wang QY, Liu JS, Hu B (2016) Integration of copper subcellular distribution and chemical forms to understand copper toxicity in apple trees. Environ Exp Bot 123:125–131.
Wang J, Ye S, Xue SG, Hartley W, Wu H, Shi LZ (2018) The physiological response of Mirabilis Jalapa Linn. To lead stress and accumulation. Int Biodeterior Biodegradation 128:11–14
Wierzbicka M (1999) Comparison of lead tolerance in Allium cepa with other plant species. Environ Pollut 104(1):41–52
Wu H, Wang J, Li B, Ou Y, Wang J, Shi Q, Jiang W, Liu D, Zou J (2016) Salix matsudana Koidz tolerance mechanisms to cadmium: uptake and accumulation, subcellular distribution, and chemical forms. Pol J Environ Stud 25:1739–1747
Wu Z, McGrouther K, Chen D, Wu W, Wang H (2013) Subcellular distribution of metals within Brassica chinensis L. in response to elevated lead and chromium stress. J Agric Food Chem 61:4715–4722
Xue WX, Jiang Y, Shang XS, Zou JH (2020) Characterisation of early responses in lead accumulation and localization of Salix babylonica L. roots. BMC Plant Biol 20:296
Yang J, Li K, Zheng W, Zhang H, Cao X, Lan Y, Yang C, Li C (2015) Characterization of early transcriptional responses to cadmium in the root and leaf of cd-resistant Salix matsudana Koidz. BMC Genomics 16:705
Zhao L, Li T, Yu H, Chen G, Zhang X, Zheng Z, Li J (2015) Changes in chemical forms, subcellular distribution, and thiol compounds involved in Pb accumulation and detoxification in Athyrium wardii (hook.). Environ. Sci. Pollut. Res. 22(16):12676–12688
Zhang J, Tian S, Lu L, Shohag MJI, Liao HB, Yang XE (2011) Lead tolerance and cellular distribution in Elsholtzia splendens using synchrotron radiation micro-X-ray fluorescence. J Hazard Mater 197:264–271
Zhivotovsky OP, Kuzovkina JA, Schulthess CP, Morris T, Pettinelli D, Ge M (2010) Hydroponic screening of willows (Salix L.) for lead tolerance and accumulation. International journal of phytoremediation 13(1):75–94
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31770653; 314000526) and the Zhejiang Science and Technology Major Program on Agricultural (trees) New Variety Breeding (2016C02056-11). We acknowledge the support from the 4W1B beamline of BSRF (Beijing Synchrotron Radiation Facility), Institute of High Energy Physics, Chinese Academy of Sciences. We are grately thankful for the help of Dr. Dongliang Chen and Dr. Juncai Dongfor their technical assistance in data acquisition and processing. We are also thankful to Dr. Hengfu Yin and Dr. Xiaojiao Han for their valuable suggestions during the revision period.
Author information
Authors and Affiliations
Contributions
Shufeng Wang: Conceptualization, Methodology, Original draft preparation.
Xiang Shi: Data curation.
Guangcai Chen: Conceptualization, Methodology, Supervision.
Mir Md Abdus Salam: Reviewing and Editing.
Corresponding author
Ethics declarations
Compliance with ethical standards
The authors declare that there is no conflicts of interest exists among fundings, and no Human Participants and/or Animals involved.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Juan Barcelo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary file 1
Histochemical stainning of Pb in root tips of S. integra using dithizone method in 0–48 h treated with 100 μM of Pb (PNG 26376 kb)
Supplementary file 2
Energy counts of Pb and other mutrient elements based on micro-XRF scanning (XLSX 31 kb)
ESM 3
(PDF 250 kb)
Supplementary file 4
Transmission electron micrographs of plant cells.((a): Root cells of the control plants; (b) and (c): Root cells exposed to 200 μM Pb for 14 days; (d): Leaf cells of the control plants; (e) and (f): Leaf cells exposed to 200 μM Pb for 14 days; CW: cell walls; V: vacuole; IS: intercellular spaces; TP: trophoplast; Pl: plasmalemma; ER: endoplasmic reticulum; Chl: chloroplast. 1–6: energy spectrum sites) (PNG 25010 kb)
Supplementary file 5
EDX-spectrum of Pb precipitates in different subcellular spaces of root and leaf. (1: root cell walls and intercellular spaces; 2: trophoplast; 3: endoplasmic reticulum; 4: leaf cell walls; 5: intercellular spaces of leaf cells; 6: valuoles of leaf cells.) (PNG 25010 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Shi, X., Salam, M.M.A. et al. Integrated study on subcellular localization and chemical speciation of Pb reveals root strategies for Pb sequestration and detoxification in Salix integra. Plant Soil 467, 197–211 (2021). https://doi.org/10.1007/s11104-021-05045-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05045-1