Abstract
Background
Although elevated [CO2] causes an increase of photosynthesis in the short-term, this increase is often attenuated over time due to decreased photosynthetic capacity of the leaf in a process called photosynthetic acclimation to elevated CO2 (PAC). PAC is often accompanied by N deficiency and sink:source imbalance. The aim of this study is to investigate mechanisms that lead to PAC, N deficiency and sink:source imbalance in tomato plants grown in elevated [CO2] and how they are affected by different levels of N treatments.
Methods
Two long-term experiment and two short-term experiments were conducted in which tomato plants were grown in chambers with ambient [CO2] and elevated [CO2] combined with different levels of N nutrition. The following parameters were measured: 1) Biomass 2)Leaf N, P and K concentrations, 3) leaf NO3− concentration, 4) Gas exchange 5) Rubisco expression and 6) Leaf starch concentration.
Results
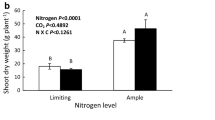
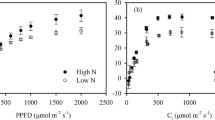
Plants grown at e[CO2] had increased biomass and starch, and decreased gas exchange, stomatal conductivity, Rubisco expression, Vcmax, NPK and leaf NO3− . Increasing N fertilization counteracted many of the effects of elevated [CO2].
Conclusions
PAC was caused by decreased N uptake or transport coupled with increased growth which leads to N deficiency and a sink:source imbalance. Increased N fertilization counteracted the effect of e[CO2] on photosynthesis, N status, and sink:source imbalance. Furthermore, elevated [CO2] caused stomata to partially close, which accounted for some of the PAC observed.










Similar content being viewed by others
Abbreviations
- e[CO2]:
-
Elevated CO2 concentration
- Ci:
-
Partial pressure of CO2 in intercellular spaces
- Co:
-
Partial pressure of CO2 outside the leaf
- LeEF-1:
-
Elongation factor gene expression
- Ls:
-
Stomatal limitation
- PAC:
-
Photosynthetic acclimation to elevated CO2
- Vcmax :
-
Maximum rate of carboxylation
References
Ainsworth E, Rogers A, Nelson R, Long S (2004) Testing the ‘source-sink’ hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in glycine max. Agric For Meteorol 112:85–94
Arp W (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875
Bailey B (2002) Tomatoes: guide for CO2 enrichment, a grower guide. Horticultural Development Council. http://www.hdc.org.uk/sites/default/files/CO2%20Optimiser.pdf. Last accessed 10.06.18
Bloom A, Smart D, Nguyen D, Searles P (2002) Nitrogen assimilation and growth of wheat under elevated CO2. PNAS 99(3):1730–1735
Bloom A, Burger M, Rubio-Asensio J, Cousins A (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903
Bloom A, Burger M, Kimball B, Pinter P (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Chang 4(6):477–480
Dlugokencky E, Tans P (2017) Trends in Atmospheric Carbon Dioxide. NOAA/ESRL. www.esrl.noaa.gov/gmd/ccgg/trends/. Last accessed 01.01.18
Erel R, Ben-Gal A, Dag A, Schwartz A, Yermiyahu U (2014) Sodium replacement of potassium in physiological processes of olive trees (Var. Barnea) as affected by drought. Tree Physiol 34(10):1102–1117
Erel R, Yermiyahu U, Ben-Gal A, Dag A, Shapira O, Schwartz A (2015) Modification of non-stomatal limitation and Photoprotection due to K and Na nutrition of olive trees. J Plant Physiol 177:1–10
Ethier GJ, Livingston (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-berry leaf photosynthesis model. Plant Cell Environ 27(2):137–153
FAO (2016) FAOSTAT, http://www.fao.org/faostat/en/#data/QC, last accessed 1.06.18
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33(1):317–345
Farquhar GD, Caemmerer S, Berry J (1980) A biochemical-model of photosynthetic CO2 assimilation in eaves of C-3 species. Planta 149(1):78–90
Ferrario-Méry S, Thibaud M, Betsche T, Valadier M, Foyer C (1997) Modulation of carbon and nitrogen metabolism, and of nitrate reductase, in untransformed and transformed Nicotiana Plumbaginifolia during CO2 enrichment of plants grown in pots and in hydroponic culture. Planta 202(4):510–521
Fisher J, Tu K (2013) A Ci Curve Fitting 10.0. http://landflux.org/Tools.php. Last accessed 17.6.18
Geiger M, Walch-Liu P, Engels C, Harnecker J, Schulze E, Ludewig F, Sonnewald U, Scheible W, Stitt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21(3):253–268
Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M (1999) The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ 22:1177–1199
Griffin K, Seemann J (1996) Plants, CO2 and photosynthesis in the 21st century. Chem Biol 3(4):245–254
Gunderson C, Wullschleger S (1994) Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photosynth Res 39:369–388
Hepworth C, Doheny-Adams T, Hunt L, Cameron D (2015) Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol 208:336–341
IPCC (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp
Jauregui I, Aroca R, Garnica M, Zamarreño A, García-Mina J, Serret M Parry M, Irigoyen J, Aranjuelo I (2015) Nitrogen assimilation and transpiration: key processes conditioning responsiveness of wheat to elevated [CO2] and temperature. Physiol Plant 155(3):338–354
Juan L, Zhou J, Duan Z (2007) Effects of elevated CO2 concentration on growth and water usage of tomato seedlings under different ammonium/nitrate ratios. J Environ Sci 19:1100–1107
Kruse J, Hetzger I, Hänsch R, Mendel R, Walch-Liu P, Engels C, Rennenberg H (2002) Elevated pCO(2) Favours nitrate reduction in the roots of wild-type tobacco (Nicotiana Tabacum cv. Gat.) and significantly alters N-metabolism in Transformants lacking functional nitrate reductase in the roots. J Exp Bot 53(379):2351–2367
Leakey A, Ainsworth E, Bernacchi C, Rogers A, Long S, Ort D (2009) Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J Exp Bot 60(10):2859–2876
Lugassi N, Kelly G, Fidel L, Yaniv Y, Attia Z, Levi A, Alchanatis V, Moshelion M, Raveh E, Carmi N, Granot D (2015) Expression of Arabidopsis hexokinase in Citrus guard cells controls stomatal aperture and reduces transpiration. Front Plant Sci 6(December):1–11
Mamatha H, Srinvasa N, Laxman R, Shivashankara K, Bhatt R, Pavithra K (2014) Impact of elevated CO2 on growth, yield, and quality of tomato (Lycopersicon esculentum mill.) CV. Arka Ashish. Photosynthetica 52(4):519–528
Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ 24(11):1119–1137
McDonald E, Erickson J, Kruger E (2002) Can decreased transpiration limit plant nitrogen Acquisition in elevated CO2? Funct Plant Biol 29(9):1115–1120
Miller G (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anall Chem 31(3):426–428
Moore B, Cheng S, Sims D, Seemann J (1999) The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ 22(6):567–582
Purvis A, Peters D, Hageman R (1974) Effect of carbon dioxide on nitrate accumulation and nitrate reductase induction in corn seedlings. Plant Physiol 53(6):934–941
Rubio-Asensio J, Bloom A (2017) Inorganic nitrogen form: a major player in wheat and Arabidopsis responses to elevated CO 2. J Exp Bot 68(10):2611–2625
Ruiz-Vera U, De Souza A, Long S, Ort D (2017) The role of sink strength and nitrogen availability in the Down-regulation of photosynthetic capacity in field-grown Nicotiana Tabacum L. at elevated CO2 concentration. Front. Plant Sci 8(Jun):1–12
Sims D, Luo Y, Seemann J (1998) Comparison of photosynthetic acclimation to elevated CO2 and limited nitrogen supply in soybean. Plant Cell Environ 21(9):945–952
Snell F, Snell C (1949) Colorimetric methods of analysis, 3rd edn. D. Van Nostran Co Inc., New York
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22(6):583–621
Taub D, Wang X (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50(11):1365–1374
Tripp K, Peet M, Willits D, Pharr D (1991) CO2 enhanced foliar deformation of tomato: relationship to foliar starch concentration. J Amer Soc Hort Sci 116(15):876–880
Van Oosten J, Besford R (1996) Acclimation of photosynthesis to elevated CO2 through feedback regulation of gene expression: climate of opinion. Photosynth Res 48(3):353–365
Van Oosten J, Wilkins D, Besford R (1994) Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by the regulation by carbohydrates: a mechanism for the acclimation of photosynthesis to high CO2. Plant Cell Environ 17:913–923
Van Oosten J, Wilkins D, Besford R (1995) Acclimation of tomato to different carbon-dioxide concentrations - relationships between biochemistry and gas-exchange during leaf development. New Phytol 130(3):357–367
Vishnevetsky J, Zamski R, Ziv M (2000) Carbohydrate metabolism in Nerine Sarniensis bulbs developing in liquid. Physiol Plant 108:361–369
Wall G, Adam N, Brooks T, Kimball B, Pinter P, Lamorte R, Adamsen F et al (2000) Acclimation response of spring wheat in a free-air CO 2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 2. Net assimilation and stomatal conductance of leaves. Photosynth Res 66:79–95
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7(May):1–15
Yelle S, Beeson R, Trudel M, Gosselin A (1989) Acclimation of two tomato species to Hihh atmospheric CO2: sugar and starch concentration. Plant Physiol 90:1465–1472
Yelle S, Beeson R, Trudel M, Gosselin A (1990) Duration of CO2 enrichment influences growth, yield, and gas exchange in two tomato species. J Amer Soc Hort Sci 115(1):52–57
Yermiyahu U, Heuer B, Silverman D, Faingold I, Avraham L (2017) Nitrate analysis of Diplotaxis Tenuifolia: fresh versus dry material for meeting international standards and regulations. Israel J Plant Sci (Mar):1–4
Acknowledgements
We would like to thank Inna Faingold, Hila Hecht-Ganan, Lital Zelnik and Dan Hamus Cohen for their help with the lab analyses. We would also like to thank Mohamed Alhosa for dealing with plant pathogens that arose during the experiments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Ad C. Borstlap.
Rights and permissions
About this article
Cite this article
Halpern, M., Bar-Tal, A., Lugassi, N. et al. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 434, 397–411 (2019). https://doi.org/10.1007/s11104-018-3857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3857-5