Abstract
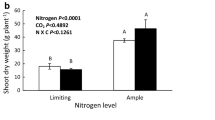
Growth in elevated CO2 often leads to decreased plant nitrogen contents and down-regulation of photosynthetic capacity. Here, we investigated whether elevated CO2 limits nitrogen uptake when nutrient movement to roots is unrestricted, and the dependence of this limitation on nitrogen supply and plant development in durum wheat (Triticum durum Desf.). Plants were grown hydroponically at two N supplies and ambient and elevated CO2 concentrations. Elevated CO2 decreased nitrate uptake per unit root mass with low N supply at early grain filling, but not at anthesis. This decrease was not associated with higher nitrate or amino acid, or lower non-structural carbohydrate contents in roots. At anthesis, elevated CO2 decreased the nitrogen content of roots with both levels of N and that of aboveground organs with high N. With low N, elevated CO2 increased N allocation to aboveground plant organs and nitrogen concentration per unit flag leaf area at anthesis, and per unit aboveground dry mass at both growth stages. The results from the hydroponic experiment suggest that elevated CO2 restricts nitrate uptake late in development, high N supply overriding this restriction. Increased nitrogen allocation to young leaves at low N supply could alleviate photosynthetic acclimation to elevated CO2.



Similar content being viewed by others
References
Ainsworth E, Long S (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371
Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ et al (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biol 8:695–709
Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric Forest Meteorol 122:85–94
Allard V, Newton PCD, Lieffering M, Soussana JF, Carran RA, Matthew C (2005) Increased quantity and quality of coarse soil organic matter fraction at elevated CO2 in a grazed grassland are a consequence of enhanced root growth rate and turnover. Plant Soil 276:49–60
Aranjuelo I, Cabrera-Bosquet L, Morcuende R, Avice JC, Nogués S, Araus JL et al (2011) Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J Exp Bot 62:3957–3969
Aranjuelo I, Sanz-Sáez Á, Jauregui I, Irigoyen JJ, Araus JL, Sánchez-Díaz M et al (2013) Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J Exp Bot 64:1879–1892
Arp WJ (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875
BassiriRad H, Prior SA, Norby RJ, Rogers HH (1999) A field method of determining NH4 + and NO3 − uptake kinetics in intact roots: effects of CO2 enrichment on trees and crop species. Plant Soil 217:195–204
BassiriRad H, Gutschick VP, Lussenhop J (2001) Root system adjustments: regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 126:305–320
Benlloch-Gonzalez M, Bochicchio R, Berger J, Bramley H, Palta JA (2014) High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crop Res 165:71–79
Bernacchi CJ, Kimball BA, Quarles DR, Long SP, Ort DR (2007) Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol 143:134–144
Berntson GM, Rajakaruna N, Bazzaz FA (1998) Growth and nitrogen uptake in an experimental community of annuals exposed to elevated atmospheric CO2. Global Change Biol 4:607–626
Bielenberg DG, BassiriRad H (2005) Nutrient acquisition of terrestrial plants in a changing climate. In: BassiriRad H (ed) Nutrient acquisition by plants. Springer, Berlin, pp 311–329
Bloom AJ, Smart DR, Nguyen DT, Searles PS, Smart D, Nguyen D et al (2002) Nitrogen assimilation and growth of wheat under elevated carbon dioxide. P Natl Acad Sci USA 99:1730–1735
Bloom AJ, Burger M, Asensio JSR, Cousins A (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903
Bloom AJ, Burger M, Kimball BA, Pinter PJ Jr (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Change 4:477–480
Bowes G (1993) Facing the inevitable: plants and increasing atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol 44:309–332
Carlisle E, Myers S, Raboy V, Bloom A (2012) The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front Plant Sci 3:195
Cawse PA (1967) The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst 92:311–315
Coleman JS, McConnaughay KDM, Bazzaz FA (1993) Elevated CO2 and plant nitrogen-use: is reduced tissue nitrogen concentration size-dependent? Oecologia 93:195–200
Crawford N, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395
De Graaff M-A, Van Groenigen K-J, Six J, Hungate B, Van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biol 12:2077–2091
Del Pozo A, Pérez P, Morcuende R, Alonso A, Martínez-Carrasco R (2005) Acclimatory responses of stomatal conductance and photosynthesis to elevated CO2 and temperature in wheat crops grown at varying levels of N supply in a Mediterranean environment. Plant Sci 169:908–916
Del Pozo A, Pérez P, Gutiérrez D, Alonso A, Morcuende R, Martínez-Carrasco R (2007) Gas exchange acclimation to elevated CO2 in upper-sunlit and lower-shaded canopy leaves in relation to nitrogen acquisition and partitioning in wheat grown in field chambers. Environ Exp Bot 59:371–380
Delgado E, Mitchell RAC, Parry MAJ, Driscoll SP, Mitchell VJ, Lawlor DW (1994) Interacting effects of CO2 concentration, temperature and nitrogen supply on the photosynthesis and composition of winter wheat leaves. Plant Cell Environ 17:1205–1213
Diaz S, Grime JP, Harris J, McPherson E (1993) Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide. Nature 364:616–617
Farage PK, McKee IF, Long SP (1998) Does a low nitrogen supply necessarily lead to acclimation of photosynthesis to elevated CO2? Plant Physiol 118:573–580
Ferrario-Méry S, Thibaud MC, Betsche T, Valadier MH, Foyer CH (1997) Modulation of carbon and nitrogen metabolism, and of nitrate reductase, in untransformed and transformed Nicotiana plumbaginifolia during CO2 enrichment of plants grown in pots and in hydroponic culture. Planta 202:510–521
Fonseca F, Bowsher CG, Stulen I (1997) Impact of elevated atmospheric CO2 on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol Plant 100:940–948
Geiger M, Walch-Liu P, Engels C, Harnecker J, Schulze ED, Ludewig F et al (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M (1999) The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ 22:1177–1199
Gesch RW, Kang IH, Gallo Meagher M, Vu JCV, Boote KJ, Allen LH et al (2003) Rubisco expression in rice leaves is related to genotypic variation of photosynthesis under elevated growth CO2 and temperature. Plant Cell Environ 26:1941–1950
Gifford R, Barrett D, Lutze J (2000) The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 224:1–14
Gutiérrez D, Morcuende R, Del Pozo A, Martínez-Carrasco R, Pérez P (2013) Involvement of nitrogen and cytokinins in photosynthetic acclimation to elevated CO2 of spring wheat. J Plant Physiol 170:1337–1343
Haase S, Neumann G, Kania A, Kuzyakov Y, Römheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39:2208–2221
Hare PE (1977) Subnanomole-range amino acid analysis. Method Enzymol 47:3–18
Havelka UD, Wittenback VA, Boyle MG (1984) CO2-enrichment effects on wheat yield and physiology. Crop Sci 24:1163–1168
Hirel B, Le Gouis J, Ney B, Gallais A (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58:2369–2387
Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB (2003) Nitrogen and climate change. Science 302:1512–1513
Jackson RB, Reynolds HL (1996) Nitrate and ammonium uptake for single- and mixed-species communities grown at elevated CO2. Oecologia 105:74–80
Johansson EM, Fransson PMA, Finlay RD, van Hees PAW (2009) Quantitative analysis of soluble exudates produced by ectomycorrhizal roots as a response to ambient and elevated CO2. Soil Biol Biochem 41:1111–1116
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Adv Agron 77:293–368
Krapp A, Fraisier V, Scheible W-R, Quesada A, Gojon A, Stitt M et al (1998) Expression studies of Nrt 2:1Np, a putative high-affinity nitrate transporter: evidence for its role in nitrate uptake. Plant J 14:723–731
Kruse J, Hetzger I, Hänsch R, Mendel RR, Walch-Liu P, Engels C et al (2002) Elevated pCO2 favours nitrate reduction in the roots of wild-type tobacco (Nicotiana tabacum cv. Gat.) and significantly alters N-metabolism in transformants lacking functional nitrate reductase in the roots. J Exp Bot 53:2351–2367
Lee TD, Tjoelker MG, Ellsworth DS, Reich PB (2001) Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytol 150:405–418
Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel Vedele F et al (1999) Molecular and functional regulation of two NO3 − uptake systems by N- and C-status of Arabidopsis plants. Plant J 18:509–519
Lekshmy S, Jain V, Khetarpal S, Pandey R (2013) Inhibition of nitrate uptake and assimilation in wheat seedlings grown under elevated CO2. Ind J Plant Physiol 18:23–29
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Makino A, Harada M, Sato T, Nakano H, Mae T (1997) Growth and N allocation in rice plants under CO2 enrichment. Plant Physiol 115:199–203
Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ 24:1119–1137
McDonald EP, Erickson JE, Kruger EL (2002) Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct Plant Biol 29:1115–1120
McMaster GS, LeCain DR, Morgan JA, Aiguo L, Hendrix DL (1999) Elevated CO2 increases wheat cer, leaf and tiller development, and shoot and root growth. J Agron Crop Sci 183:119–128
Morcuende R, Kostadinova S, Pérez P, Martín del Molino IM, Martínez-Carrasco R (2004) Nitrate is a negative signal for fructan synthesis, and the fructosyltransferase-inducing trehalose inhibits nitrogen and carbon assimilation in excised barley leaves. New Phytol 161:749–759
Nakano H, Makino A, Mae T (1997) The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol 115:191–198
Nie GY, Hendrix DL, Webber AN, Kimball BA, Long SP (1995) Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2 concentration in the field. Plant Physiol 108:975–983
Oscarson P (2000) The strategy of the wheat plant in acclimating growth and grain production to nitrogen availability. J Exp Bot 51:1921–1929
Pal M, Rao LS, Jain V, Srivastava AC, Pandey R, Raj A et al (2005) Effects of elevated CO2 and nitrogen on wheat growth and photosynthesis. Biol Plantarum 49:467–470
Pang J, Zhu J-G, Xie Z-B, Liu G, Zhang Y-L, Chen G-P et al (2006) A new explanation of the N concentration decrease in tissues of rice (Oryza sativa L.) exposed to elevated atmospheric pCO2. Environ Exp Bot 57:98–105
Pearman I, Thomas SM, Thorne GN (1978) Effect of nitrogen fertilizer on growth and yield of semi-dwarf and tall varieties of winter wheat. J Agric Sci 91:31–45
Pérez P, Martínez-Carrasco R, Sánchez de la Puente L (1983) Uptake and distribution of nitrogen in wheat plants supplied with different amounts of nitrogen after stem elongation. Ann Appl Biol 102:399–406
Pérez P, Morcuende R, Martín del Molino I, Martínez-Carrasco R (2005) Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient tunnels. Environ Exp Bot 53:13–27
Phillips DA, Fox TC, Six J (2006) Root exudation (net efflux of amino acids) may increase rhizodeposition under elevated CO2. Global Change Biol 12:561–567
Poorter H, Roumet C, Campbell BD (1996) Interspecific variation in the growth response of plants to elevated CO2: a search for functional types. In: Bazzaz CKA (ed) Carbon dioxide, populations, and communities. Academic Press, San Diego, pp 375–412
Poorter H, Van Berkel Y, Baxter R, Den Hertog J, Dijkstra P, Gifford RM et al (1997) The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ 20:472–482
Ritchie RJ (2006) Estimation of cytoplasmic nitrate and its electrochemical potential in barley roots using 13NO3 − and compartmental analysis. New Phytol 171:643–655
Rogers GS, Milham PJ, Gillings M, Conroy JP (1996) Sink strength may be the key to growth and nitrogen responses in N-deficient wheat at elevated CO2. Aust J Plant Physiol 23:253–264
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Scheible WR, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9:783–798
Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios Rojas N et al (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Schulze W, Schulze ED, Stadler J, Heilmeier H, Stitt M, Mooney HA (1994) Growth and reproduction of Arabidopsis thaliana in relation to storage of starch and nitrate in the wild-type and in starch-deficient and nitrate-uptake-deficient mutants. Plant Cell Environ 17:795–809
Shimono H, Bunce JA (2009) Acclimation of nitrogen uptake capacity of rice to elevated atmospheric CO2 concentration. Ann Bot 103:87–94
Smart DR, Ritchie K, Bloom AJ, Bugbee BB (1998) Nitrogen balance for wheat canopies (Triticum aestivum cv Veery 10) grown under elevated and ambient CO2 concentrations. Plant Cell Environ 21:753–763
Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2:178
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R et al (2002) Steps towards an integrated view of nitrogen metabolism. J Exp Bot 53:959–970
Taub DR, Wang X (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374
Taub DR, Miller B, Allen H (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol 14:565–575
Thorne GN (1974) Physiology of grain yield of wheat and barley. Report of Rothamsted experimental station for 1973, Part 2, 5–25
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zak D, Pregitzer K, Curtis P, Teeri J, Fogel R, Randlett D (1993) Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151:105–117
Acknowledgments
This work has been funded by the Spanish National Research and Development Programme-European Regional Development Fund, ERDF (grant no. AGL2009-11987). R.V. was the recipient of a fellowship with reference BES-2010-031029. We are grateful to Dr J.M. Igual for his critical review of the manuscript. The technical cooperation of A. Verdejo and M.A. Boyero in control of hydroponic cultures, harvesting and N measurements is acknowledged.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2015_1867_MOESM1_ESM.tif
Supplementary Fig. 1 Mean daily courses of temperature, relative humidity and CO2 concentration in controlled environment chambers used for ambient (white circles) or elevated (black circles) [CO2] treatments. Records correspond to hourly averages ± standard deviation for the duration of the whole experiment(TIFF 440 kb)
Rights and permissions
About this article
Cite this article
Vicente, R., Pérez, P., Martínez-Carrasco, R. et al. Nitrate supply and plant development influence nitrogen uptake and allocation under elevated CO2 in durum wheat grown hydroponically. Acta Physiol Plant 37, 114 (2015). https://doi.org/10.1007/s11738-015-1867-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1867-y