Abstract
Background and aims
The olive root endophyte Pseudomonas fluorescens PICF7 is an effective biocontrol agent of Verticillium wilt of olive (VWO). Colonization of olive roots either by strain PICF7 or by Verticillium dahliae triggers differential systemic transcriptomic responses, many of them related with defense-related genes. The aims were to develop an olive split-root system for assessing VWO development and biocontrol effectiveness of strain PICF7 in plants with a divided root architecture, and for evaluating systemic defense responses during this tripartite interaction when strain PICF7 and V. dahliae are spatially separated.
Methods
An olive split-root system was generated and disease development, biocontrol effectiveness and systemic genetic responses in these plants upon strain PICF7 and V. dahliae colonization were compared to those reported and observed in olive plants grown under standard conditions (single pots). Specific defense-related genes, previously identified during PICF7- and/or V. dahliae-olive root interactions were selected and their expression patterns assessed in above-ground tissues by real-time qPCR analyses.
Results
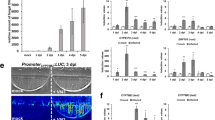
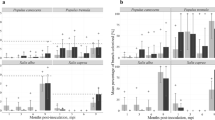
Symptoms of VWO developed similarly both in split-root and single-root plants. However, even though PICF7 triggered systemic defense responses in aerial tissues prior to the infection by V. dahliae, effective biocontrol was not observed under these experimental conditions. While most of studied genes showed similar expression patterns along time in both systems (i.e. split root and single pot), some of them (e.g. the caffeoyl-O-methyltransferase coding gene) varied depending on whether strain PICF7 and V. dahliae were spatially separated or shared the same compartment.
Conclusions
A successful split-root system was generated to investigate genetic events taking place during the tripartite interaction olive-V. dahliae-P. fluorescens PICF7. VWO biocontrol by strain PICF7 must rely on mechanisms other than induction of systemic resistance responses. The expression pattern of specific defense-related olive genes depended on whether or not the biocontrol agent and the pathogen share the same root/soil region.




Similar content being viewed by others
References
Aganchich B, Tahi H, Wahbi S, Elmodaffar C, Serraj R (2007) Growth, water relations and antioxidant defence mechanisms of olive (Olea europaea L.) subjected to partial root drying (PRD) and regulated deficit irrigation (RDI). Plant Biosyst 141:252–264
Aimé S, Alabouvette C, Steinberg C, Olivain C (2013) The endophytic strain Fusarium oxysporum Fo47: a good candidate for priming the defense responses in tomato roots. Mol Plant-Microbe Interact 26:918–926
Ali A, Ahmad T, Abbasi NA, Hafiz IA (2009) Effect of different concentrations of auxins on in vitro rooting of olive cultivar ‘Moraiolo’. Pak J Bot 41:1223–1231
Bashan Y, de-Bashan LE, Prabhu SR, Hernández J-P (2013) Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant Soil 378:1-33
Bordiec S, Paquis S, Lacroix H, Dhondt S, Barka EA, Kauffmann S, Jeandet P, Mazeyrat-Gourbeyre F, Clément C, Baillieul F, Dorey S (2011) Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J Exp Bot 62:595–603
Boukcim H, Pagès L, Mousain D (2006) Local NO3 − or NH4 + supply modifies the root system architecture of Cedrus atlantica seedlings grown in a split-root device. J Plant Physiol 163:1293–1304
Bourquin V, Nishikubo N, Abe H, Brumer H, Denman S, Eklund M, Christiernin M, Teeri TT, Sundberg B, Mellerowicz EJ (2002) Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. Plant Cell 14:3073–3088
Butterfield EJ, Devay JE (1977) Reassessment of soil assays for Verticillium dahliae. Phytopathology 67:1073–1078
Caballero JM, Del Río C (2010) Propagation methods. In: Barranco D, Fernández-Escobar R, Rallo L (eds) Olive growing. Junta de Andalucía/Mundi Prensa/RIRDC/AOA, Pendle Hill, pp 83–112
Campbell CL, Madden LV (1990) Introduction to plant disease Epidemiology. John Wiley & Sons, New York
Carder JH, Morton A, Tabrett AM, Barbara DJ (1994) Detection and differentiation by PCR of subspecific groups within two Verticillium species causing vascular wilts in herbaceous hosts. In: Schots A, Dewey FM, Oliver R (eds) Modern assays for plant pathogenic fungi. CAB International, Oxford, pp 91–97
Cazorla FM, Mercado-Blanco J (2016) Biological control of tree and woody plant diseases: an impossible task? Biol Control 61:233–242
Chi Y, Yang Y, Zhou Y, Zhou J, Fan B, Yu JQ, Chen Z (2013) Protein-protein interactions in the regulation of WRKY transcription factors. Mol Plant 6:287–300
Ciancio A, Pieterse CMJ, Mercado-Blanco J (2016) Editorial: harnessing useful rhizosphere microorganisms for pathogen and Pest biocontrol. Front Microbiol 7:1620
Conn VM, Walker AR, Franco CM (2008) Endophytic actinobacteria induces defense pathways in Arabidopsis thaliana. Mol Plant-Microbe Interact 21:208–218
Cwalina-Ambroziak B, Nowak M (2012) The effects of biological and chemical controls on fungal communities colonising tomato (Lycopersicon esculentum mill.) plants and soil. Folia Hort 24:13–20
Dbara S, Haworth M, Emiliani G, Ben Mimoun M, Gómez-Cadenas A, Centritto M (2016) Partial root-zone drying of olive (Olea europaea var. ‘Chetoui’) induces reduced yield under field conditions. PLoS ONE 11:e0157089
Dry PR, Loveys BR (1999) Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis 38:151–156
Gafni A, Calderon CE, Harris R, Buxdorf K, Dafa-Berger A, Zeilinger-Reichert E, Levy M (2015) Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front Plant Sci 6:132
Gallou A, Cranenbrouck S, Declerck S (2009) Trichoderma harzianum elicits defence response genes in roots of potato plantlets challenged by Rhizoctonia solani. Eur Plant Pathol 124:219–230
García-Tejera O, López-Bernal A, Testi L, Villalobos FJ (2017) A soil-plant-atmosphere continuum (SPAC) model for simulating tree transpiration with a soil multi-compartment solution. Plant Soil 412:215–233
Glick BR, Karaturovíc DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536
Gómez-Lama Cabanás C, Schilirò E, Valverde-Corredor A, Mercado-Blanco J (2014) The biocontrol endophytic bacterium Pseudomonas fluorescens PICF7 induces systemic defense responses in aerial tissues upon colonization of olive roots. Front Microbiol 5:427
Gómez-Lama Cabanás C, Schilirò E, Valverde-Corredor A, Mercado-Blanco J (2015) Systemic responses in a tolerant olive (Olea europaea L.) cultivar upon root colonization by the vascular pathogen Verticillium dahliae. Front Microbiol 6:928
He CY, Hsiang T, Wolyn DJ (2002) Induction of systemic disease resistance and pathogen defence responses in Asparagus officinalis inoculated with nonpathogenic strains of Fusarium oxysporum. Plant Pathol 51:225–230
Hiemstra JA, Harris DC (eds) (1998) A compendium of Verticillium wilts in tree species. Wageningen, Ponsen & Looijen
Huisman OC, Ashworth LJ (1974) Quantitative assessment of Verticillium albo-atrum in field soils: procedural and substrate improvements. Phytopathology 64:1043–1044
Karajeh MR, Masoud SA (2006) Molecular detection of Verticillium dahliae Kleb. in asymptomatic olive trees. J Phtopathol 154:496–499
Keykhasaber M, Pham KTK, Thomma BPHJ, Hiemstra JA (2016) Reliable detection of unevenly distributed Verticillium dahliae in diseased olive trees. Plant Pathol. doi:10.1111/ppa.12647
Kim JH, Yu J, Mahoney N, Chan KL, Molyneux RJ, Varga J, Bhatnagar D, Cleveland TE, Nierman WC, Campbell BC (2008) Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int J Food Microbiol 122:49–60
Kjellin J (2012) Role of WRKY20 transcription factor and raffinose in plant defense responses upon Bacillus amyloliquefaciens strain 5113-mediated priming in Arabidopsis thaliana. Bachelor’sThesis. Dept. of Plant Biology and Forest Genetics University of Uppsala, Kjellin
Kloepper JW, Ryu C-M, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Lakshmanan V, Selvaraj G, Bais HP (2014) Functional soil microbiome: belowground solutions to an aboveground problem. Plant Physiol 166:689–700
Lavee S (1996) Biology and physiology of the olive. In: World olive encyclopedia. International Olive Oil Council, Madrid, pp 59–110
Levin AG, Lavee S, Tsror L (2003) Epidemiology of Verticillium dahliae on olive (Picual) and its effect on yield under saline conditions. Plant Pathol 52:212–218
Li H, Li H, Bai Y, Wang J, Nie M, Li B, Xiao M (2011) The use of Pseudomonas fluorescens P13 to control sclerotinia stem rot (Sclerotinia sclerotiorum) of oilseed rape. J Microbiol 49:884–889
Li Y, Yanagi A, Miyawaki Y, Okada T, Matsubara Y-I (2010) Disease tolerance and changes in antioxidative abilities in mycorrhizal strawberry plants. J Jpn Soc Hortic Sci 79:174–178
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt. Methods 25:402–408
López-Escudero FJ, del Río C, Caballero JM, Blanco-López MA (2004) Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur J Plant Pathol 110:79–85
López-Escudero FJ, Mercado-Blanco J (2011) Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 344:1–50
Maldonado-González MM, Bakker PAHM, Prieto P, Mercado-Blanco J (2015a) Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front Microbiol 6:266
Maldonado-González MM, Prieto P, Ramos C, Mercado-Blanco J (2013) From the root to the stem: interaction between the biocontrol root endophyte Pseudomonas fluorescens PICF7 and the pathogen Pseudomonas savastanoi NCPPB3335 in olive knots. Microb Biotechnol 6:275–287
Maldonado-González MM, Schilirò E, Prieto P, Mercado-Blanco J (2015b) Endophytic colonization and biocontrol performance of Pseudomonas fluorescens PICF7 in olive (Olea europaea L.) are determined neither by pyoverdine production nor swimming motility. Environ Microbiol 17:3139–3153
Manosalva PM, Bruce M, Leach JE (2011) Rice 14-3-3 protein (GF14e) negatively affects cell death and disease resistance. Plant J 68:777–787
Martínez-García PM, Ruano-Rosa D, Schilirò E, Prieto P, Ramos C, Rodríguez-Palenzuela P, Mercado-Blanco J (2015) Complete genome sequence of Pseudomonas fluorescens strain PICF7, an indigenous root endophyte from olive (Olea europaea L.) and effective biocontrol agent against Verticillium dahliae. Stand Genomic Sci 10:10
Martínez-Medina A, Fernandez I, Lok GB, Pozo MJ, Pieterse CM, Van Wees S (2016) Shifting from priming of salicylic acid to jasmonic acid regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol 213:1363–1377
Martos-Moreno C, López-Escudero FJ, Blanco-López MA (2006) Resistance of olive cultivars to the defoliating isolate of Verticillium dahliae. Hortscience 41:1–4
Mayo S, Cominelli E, Sparvoli F, González-López O, Rodríguez-González A, Gutiérrez S, Casquero PA (2016) Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum–Rhizoctonia solani interaction. Front Plant Sci 7:1109
Mejía LC, Herre EA, Sparks JP, Winter K, García MN, Van Bael SA, Stitt J, Shi Z, Zhang Y, Guiltinan MJ, Maximova SN (2014) Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol 5:479
Mercado-Blanco J, Lugtenberg BJJ (2014) Biotechnological applications of bacterial endophytes. Curr Biotechnol 3:60–75
Mercado-Blanco J, Rodríguez-Jurado D, Hervás A, Jiménez-Díaz RM (2004) Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol Control 30:474–486
Mercado-Blanco J, Rodríguez-Jurado D, Parrilla-Araujo S, Jiménez-Díaz RM (2003) Simultaneous detection of the defoliating and nondefoliating Verticillium dahliae pathotypes in infected olive plants by duplex, nested polymerase chain reaction. Plant Dis 87:1487–1494
Mercado-Blanco J, Rodríguez-Jurado D, Pérez-Artés E, Jiménez-Díaz RM (2002) Detection of the defoliating pathotype of Verticillium dahliae pathotype in infected olive plants by nested PCR. Eur J Plant Pathol 108:1–13
Mgbeahuruike AC, Kovalchuk A, Ubhayasekera W, Nelson DR, Yadav JS (2017) CYPome of the conifer pathogen Heterobasidion irregulare: inventory, phylogeny, and transcriptional analysis of the response to biocontrol. Fungal Biol 121(2):158–171
Mishra PK, Mishra S, Selvakumar G, Bisht JK, Kundu S, Gupta HS (2009) Co-inoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.) World J Microbiol Biotechnol 25:753–761
Narisawa K, Kawamata H, Currah RS, Hashiba T (2002) Suppression of Verticillium wilt in eggplant by some fungal root endophytes. Eur J Plant Pathol 108:103–109
Palazzini JM, Dunlap CA, Bowman MJ, Chulze SN (2016) Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: genome sequencing and secondary metabolite cluster profiles. Microbiol Res 192:30–36
Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150:1648–1655
Pegg GF, Brady BL (2002) Verticillium wilts. CABI Publishing International, Wallingford
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375
Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcón-Aguilar C (2002) Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J Exp Bot 53:525–534
Prieto P, Mercado-Blanco J (2008) Endophytic colonization of olive roots by the biocontrol strain Pseudomonas fluorescens PICF7. FEMS Microbiol Ecol 64:297–306
Prieto P, Navarro-Raya C, Valverde-Corredor A, Amyotte SG, Dobinson KF, Mercado-Blanco J (2009) Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb Biotechnol 2:499–511
Prieto P, Schilirò E, Maldonado-González MM, Valderrama R, Barroso-Albarracín JB, Mercado-Blanco J (2011) Root hairs play a key role in the endophytic colonization of olive roots by Pseudomonas spp. with biocontrol activity. Microb Ecol 62:435–445
Ray DL, Johnson JC (2014) Validation of reference genes for gene expression analysis in olive (Olea europaea) mesocarp tissue by quantitative real-time RT-PCR. BMC Res Notes 7:304
Roberts MR, Salinas J, Collinge DB (2002) 14-3-3 proteins and the response to abiotic and biotic stress. Plant Mol Biol 50:1031–1039
Rudrappa T, Biedrzycki ML, Kunjeti SG, Donofrio NM, Czymmek KJ, Paré PW, Bais HP (2010) The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun Integr Biol 3:130–138
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556
Schilirò E, Ferrara M, Nigro F, Mercado-Blanco J (2012) Genetic responses induced in olive roots upon colonization by the biocontrol endophytic bacterium Pseudomonas fluorescens PICF7. PLoS One. doi:10.1371/journal.pone.0048646
Shan XC, Goodwin PH (2006) Silencing an ACC oxidase gene affects the susceptible host response of Nicotiana benthamiana to infection by Colletotrichum orbiculare. Plant Cell Rep 25:241–247
Sharmin S, Azam MS, Islam MS, Sajib AA, Mahmood N, Hasan AM, Ahmed R, Sultana K, Khan H (2012) Xyloglucan endotransglycosylase/hydrolase genes from a susceptible and resistant jute species show opposite expression pattern following Macrophomina phaseolina infection. Commun Integr Biol 5:598–606
Siddiqui IA, Shaukat SS (2002) Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. J Phytopathol 150:469–473
Tsror L (2011) Epidemiology and control of Verticillium wilt on olive. Isr J Plant Sci 59:59–69
Varo A, Mulero-Aparicio A, Adem M, Roca LF, Raya-Ortega MC, López-Escudero FJ, Trapero A (2017) Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop Prot 92:168–175
Wang L, Shi H, Wu J, Cao F (2016) Alternative partial root-zone irrigation enhances leaf flavonoid accumulation and water use efficiency of Ginkgo biloba. New For 47:377–391
Wu QS, Cao MQ, Zou YN, Wu C, He XH (2016) Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci Hortic 199:95–102
Zheng Z, Qamar SA, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48:592–605
Acknowledgements
Research Supported by grant P12-AGR667 from Junta de Andalucía (Spain) and grants AGL2009-07275 and AGL2011-30137 from the Spanish MICINN/MINECO, all co-financed by the European Regional Development Fund (ERDF) of the European Union (EU). Thanks are due to Dr. Mario Pérez Rodríguez for his excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Carmen Gómez-Lama Cabanás and Rafael Sesmero are joint first authors.
Electronic supplementary material
Table S1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Cabanás, C.GL., Sesmero, R., Valverde-Corredor, A. et al. A split-root system to assess biocontrol effectiveness and defense-related genetic responses in above-ground tissues during the tripartite interaction Verticillium dahliae-olive-Pseudomonas fluorescens PICF7 in roots. Plant Soil 417, 433–452 (2017). https://doi.org/10.1007/s11104-017-3269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3269-y