Abstract
Key message
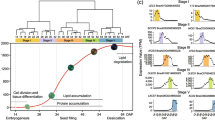
In this manuscript, we explored the key molecular networks for oil biosynthesis with the transcriptome and metabolome of B. napus embryo at different developmental stages.
Abstract
Brassica napus (B. napus) is an important oil crop worldwide, yet the molecular pathways involved in oil biosynthesis in seeds are not fully understood. In this study, we performed a combined investigation of the gene expression profiles and metabolite content in B. napus seeds at 21, 28 and 35 days after flowering (DAF), when seed oil biosynthesis takes place. The total triacylglycerol (TAG) content in seed embryos increased over the course of seed maturation, and was accompanied by changes in the fatty acid profile, an increase in lipid droplets, and a reduction in starch grains. Metabolome analysis showed that the total amino acid, free fatty acid and organic acid contents in seed embryos decreased during seed maturation. In total, the abundance of 76 metabolites was significantly different between 21 and 28 DAF, and 68 metabolites changed in abundance between 28 and 35 DAF. Transcriptome analysis showed that the set of genes differentially expressed between stages was significantly enriched in those related to lipid metabolism, transport, protein and RNA metabolism, development and signaling, covering most steps of plant lipid biosynthesis and metabolism. Importantly, the metabolite and gene expression profiles were closely correlated during seed development, especially those associated with TAG and fatty acid biosynthesis. Further, the expression of major carbohydrate metabolism-regulating genes was closely correlated with carbohydrate content during seed maturation. Our results provide novel insights into the regulation of oil biosynthesis in B. napus seeds and highlights the coordination of gene expression and metabolism in this process.






Similar content being viewed by others
References
Arondel V, Lemieux B, Hwang I, Gibson S, Goodman HM, Somerville CR (1992) Map-based cloning of a gene controlling omega-3 fatty acid desaturation in arabidopsis. Science 258:1353–1355
Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B (2010) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50:825–838
Beaudoin F, Lacey DJ, Napier JA (1999) The biogenesis of the plant seed oil body: Oleosin protein is synthesised by ER-bound ribosomes. Plant Physiol Biochem 37:481–490
Beisson F, Koo AJK, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132:681–697
Bocianowski J, Mikoإéajczyk K, Bartkowiak-Broda I (2012) Determination of fatty acid composition in seed oil of rapeseed (Brassica napus L.) by mutated alleles of the FAD3 desaturase genes. J Appl Genet 53:27–30
Chalhoub B, Denoeud F, Liu S, Parkin IA, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, Correa M, Da Silva C, Just J, Falentin C, Koh CS, Le Clainche I, Bernard M, Bento P, Noel B, Labadie K, Alberti A, Charles M, Arnaud D, Guo H, Daviaud C, Alamery S, Jabbari K, Zhao M, Edger PP, Chelaifa H, Tack D, Lassalle G, Mestiri I, Schnel N, Le Paslier MC, Fan G, Renault V, Bayer PE, Golicz AA, Manoli S, Lee TH, Thi VH, Chalabi S, Hu Q, Fan C, Tollenaere R, Lu Y, Battail C, Shen J, Sidebottom CH, Wang X, Canaguier A, Chauveau A, Berard A, Deniot G, Guan M, Liu Z, Sun F, Lim YP, Lyons E, Town CD, Bancroft I, Wang X, Meng J, Ma J, Pires JC, King GJ, Brunel D, Delourme R, Renard M, Aury JM, Adams KL, Batley J, Snowdon RJ, Tost J, Edwards D, Zhou Y, Hua W, Sharpe AG, Paterson AH, Guan C, Wincker P (2014) Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345:950–953
Chen W, Chi Y, Taylor NL, Lambers H, Finnegan PM (2010) Disruption of ptLPD1 or ptLPD2, genes that encode isoforms of the plastidial lipoamide dehydrogenase, confers arsenate hypersensitivity in Arabidopsis. Plant Physiol 153:1385–1397
Dawidowicz-Grzegorzewska A, Podstolski A (1992) Age-related changes in the ultrastructure and membrane properties of Brassica napus L. seeds. AoB Plants 69:39–46
Ekman Å, Hayden DM, Dehesh K, Bülow L, Stymne S (2008) Carbon partitioning between oil and carbohydrates in developing oat (Avena sativa L.) seeds. J Exp Bot 59:4247–4257
Flakelar CL, Prenzler PD, Luckett DJ, Howitt JA (2017) A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem 214:147–155
Flores T, Karpova O, Su X, Zeng P, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ (2008) Silencing of GmFAD3 gene by siRNA leads to low alpha-linolenic acids (18:3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)]. Transgenic Res 17:839–850
Hannoufa A, Pillai BVS, Chellamma S (2014) Genetic enhancement of Brassica napus seed quality. Transgenic Res 23:39
Harper AL, Trick M, Higgins J, Fraser F, Clissold L, Wells R, Hattori C, Werner P, Bancroft I (2012) Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nat Biotechnol 30:798–802
Hu Y, Wu G, Cao Y, Wu Y, Xiao L, Li X, Lu C (2009) Breeding response of transcript profiling in developing seeds of Brassica napus. BMC Mol Biol 10:49
Hua W, Li RJ, Zhan GM, Liu J, Li J, Wang XF, Liu GH, Wang HZ (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69:432
Hua S, Chen ZH, Zhang Y, Yu H, Lin B, Zhang D (2014) Chlorophyll and carbohydrate metabolism in developing silique and seed are prerequisite to seed oil content of Brassica napus L. Bot Stud 55:34
Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126:861
Li Z, Cheng Y, Cui J, Zhang P, Zhao H, Hu S (2015) Comparative transcriptome analysis reveals carbohydrate and lipid metabolism blocks in Brassica napus L. male sterility induced by the chemical hybridization agent monosulfuron ester sodium. BMC Genom 16:206
Li Z, Hua S, Zhang D, Yu H, Zhang Y, Lin B, Jiang L (2016) Comparison on the carbohydrate metabolic enzyme activities and their gene expression patterns in canola differing seed oil content. Plant Growth Regul 78:357–369
Malik MR, Wang F, Dirpaul JM, Zhou N, Polowick PL, Ferrie AMR, Krochko JE (2007) Transcript profiling and identification of molecular markers for early microspore embryogenesis in Brassica napus. Plant Physiol 144:134–154
Mandal S, Yadav S, Singh R, Begum G, Suneja P, Singh M (2002) Correlation studies on oil content and fatty acid profile of some Cruciferous species. Genet Resour Crop Evol 49:551–556
Niu Y, Wu G-Z, Ye R, Lin W-H, Shi Q-M, Xue L-J, Xu X-D, Li Y, Du Y-G, Xue H-W (2009) Global analysis of gene expression profiles in Brassica napus developing seeds reveals a conserved lipid metabolism regulation with Arabidopsis thaliana. Mol Plant 2:1107–1122
Ohlrogge J, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon JP, Rouster J, Paul W, Rogowsky PM (2011) Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol 156:674–686
Rakopoulos DC, Rakopoulos CD, Giakoumis EG (2015) Impact of properties of vegetable oil, bio-diesel, ethanol and n-butanol on the combustion and emissions of turbocharged HDDI diesel engine operating under steady and transient conditions. Fuel 156:1–19
Rathke GW, Behrens T, Diepenbrock W (2006) Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): a review. Agric Ecosyst Environ 117:80–108
Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997) Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol 113:75
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378
Schwender J, Ohlrogge JB (2002) Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol 130:347
Semenkovich CF (1997) Regulation of fatty acid synthase (FAS). Prog Lipid Res 36:43–53
Slocombe SP, Piffanelli P, Fairbairn D, Bowra S, Hatzopoulos P, Tsiantis M, Murphy DJ (1994) Temporal and tissue-specific regulation of a Brassica napus stearoyl-acyl carrier protein desaturase gene. Plant Physiol 104:1167–1176
Tan H, Yang X, Zhang F, Zheng X, Qu C, Mu J, Fu F, Li J, Guan R, Zhang H (2011) Enhanced seed oil production in canola by conditional expression of Brassica napus LEAFY COTYLEDON1 and LEC1-LIKE in developing seeds. Plant Physiol 156:1577–1588
Tan H, Xie Q, Xiang X, Li J, Zheng S, Xu X, Guo H, Ye W (2015) Dynamic metabolic profiles and tissue-specific source effects on the metabolome of developing seeds of Brassica napus. PLoS ONE 10:e0124794
Tan H, Xiang X, Tang J, Wang X (2016) Nutritional functions of the funiculus in Brassica napus seed maturation revealed by transcriptome and dynamic metabolite profile analyses. Plant Mol Biol 92:539–553
Turnham E, Northcote DH (1983) Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem J 212:223–229
Voelker T, Kinney AJ (2001) Variations in the biosynthesis of seed-storage lipids. Annu Rev Plant Physiol Plant Mol Biol 52:335
Wang C, Hai J, Yang J, Tian J, Chen W, Chen T, Luo H, Wang H (2016) Influence of leaf and silique photosynthesis on seeds yield and seeds oil quality of oilseed rape (Brassica napus L.). Eur J Agron 74:112–118
Woodfield HK, Sturtevant D, Borisjuk L, Munz E, Guschina IA, Chapman K, Harwood JL (2017) Spatial and temporal mapping of key lipid species in Brassica napus seeds. Plant Physiol 173:1998–2009
Woodfield HK, Cazenave-Gassiot A, Haslam RP, Guschina IA, Wenk MR, Harwood JL (2018) Using lipidomics to reveal details of lipid accumulation in developing seeds from oilseed rape (Brassica napus L.). Biochim Biophys Acta Mol Cell Biol Lipids 1863:339–348
Zou J, Katavic V, Giblin EM, Barton DL, Mackenzie SL, Keller WA, Hu X, Taylor DC (1997) Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase (SLC1-1) gene. Plant Cell 9:909
Acknowledgements
This work was supported by the NSFC Project (31671730), the National Key R&D Program of China (2016YFD0100506) and the Fundamental Research Funds for the Central Universities (KYZ201301 and KJSY201510).
Author information
Authors and Affiliations
Contributions
TH, XX, QX, SX, WX, LX, SX and ZJ carried out the experiments. TH drafted the manuscript. TH and XX conceived and designed the study and finalized the manuscript. All the authors have read and approved the final manuscript.
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, H., Zhang, J., Qi, X. et al. Correlation analysis of the transcriptome and metabolome reveals the regulatory network for lipid synthesis in developing Brassica napus embryos. Plant Mol Biol 99, 31–44 (2019). https://doi.org/10.1007/s11103-018-0800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0800-3