Abstract
The plant family Caryophyllaceae, commonly known as the pink family, is divided into 3 subfamilies and contains over 80 genera with more than 2600 species that are widely distributed in temperate climate zones. Plants belonging to this family produce a variety of secondary metabolites important in an ecological context; however, some of these metabolites also show health-promoting activities. The most important classes of phytochemicals include saponins, phytoecdysteroids, other sterols, flavonoids, lignans, other polyphenols, essential oils, and N-containing compounds such as vitamins, alkaloids or cyclopeptides. Flavonoids are polyphenolic compounds that remain one of the most extensively studied constituents of the Caryophyllaceae family. Numerous structurally diverse aglycones, including flavones, flavonols, flavonones (dihydroflavones), flavonols, isoflavones, and their O- or C-glycosides, exhibit multiple interesting biological and pharmacological activities, such as antioxidant, anti-inflammatory, anti-oedemic, antimicrobial, and immunomodulatory effects. Thus, this review analysed the flavonoid composition of 26 different genera and more than 120 species of Caryophyllaceae for the first time.
Similar content being viewed by others
Introduction
The Caryophyllaceae family, commonly known as the pink family, contains over 80 genera with more than 2600 species. The pink family is divided into 3 subfamilies, Paronychioideae, Alsinoideae, and Caryophylloideae, according to the presence or absence of stipules as well as the type of calyx and corolla. Plants of the Caryophyllaceae family are erect, prostrate, annual or perennial herbs or shrubs with simple cross-opposite leaves and swollen nodes. Tetramerous or pentamerous flowers are frequently gathered in panicle, raceme, or capitulum inflorescences (Hegnauer 1964; Kubitzki 1993; Schweingruber 2007).
The subfamily Paronychoideae, containing the genera Spergula L., Spergularia Presl., Polycarpon L. Herniaria L., and Paronychia Mill., occurs mostly in warm and tropical parts of the world. The characteristic attributes of these plants are leaves with stipules and visible separation of calyx from the corolla. The lack of stipules and the unique corolla are typical for members of the subfamily Alsinoideae: The genera Scleranthus L., Arenaria L., Sagina L., Cerastium L., Minuartia L., Stellaria L., and Colobanthus Bartl. are widespread on all continents and are even present in Antarctica. Several species of the subfamily Caryophylloideae are field weeds that inhabit northern temperate climate regions. The specific structures of this subfamily are long calyx tubes that occur in Agrostemma L., Maleandrium Roehl., Silene Mill., Gypsophila L., and Dianthus L. (the largest genus). A great number of Caryophyllaceae species are grown as decorative landscape plants. Furthermore, many members of this family produce secondary metabolites with medicinal properties (Brockington et al. 2011; Volodin and Volodina 2015).
Diversity of phytochemicals in Caryophyllaceae
Caryophyllaceae are known to be a rich source of pharmacologically active secondary metabolites spanning several structural chemical classes. Secondary metabolites are important for plants as protective chemicals against herbivores (insects, molluscs, vertebrates) and microbial pathogens (fungi, bacteria, viruses), UV light, and other plants competing for light, water, and nutrients. In addition, many secondary metabolites serve as signalling compounds to attract pollinating and seed-dispersing animals and provide communication signals among plants and symbiotic microbes (Wink 2011).
The main secondary metabolites of Caryophyllaceae are saponins, phytoecdysteroids, other sterols, flavonoids, lignans, other polyphenols, essential oils, and N-containing compounds such as vitamins, alkaloids and cyclic peptides.
Methodology
The search strategy helps to define appropriate search string and identify the relevant thematic databases to collect the relevant scientific literature. The search databases for this review were SCOPUS, PubMed/MEDLINE, Web of Science (SCI-EXPANDED), Wiley Online Library, Taylor & Francis Online, Google Scholar, REAXYS Database, Science Direct/ELSEVIER, and EBSCO Discovery Service (EDS). They have been searched systematically for articles published from 1950 until 2020. The following syntax was used: TITLE-ABS-KEY as additional search engine in combinations of the above keywords like “Caryophyllaceae”, OR “genus” (each genus from the Caryphyllaceae family was introduced), OR “phenolic compounds”, OR “flavonoids”, OR “flavones”, OR “flavonols”, OR “flavonones”, OR “isoflavones”, OR C-flavonoids”, OR “Caryophyllaceae”, OR “saponins”, OR “phytoecdysteroids”, OR “essential oils”, OR “volatile compounds”, OR “sterols”, OR “N-containing compounds”, OR “alkaloids”, OR “cyclic peptides”, OR “vitamins”, OR “lignans”, OR “bioavailability”, OR “metabolism”, OR “biological activity”. Search terms had run in separate or with limited combinations that considered the requirements, or limitations, of the database used. Additionally, based on USDA Plant Database and Kew Science (Royal Botanic Gardens), we have been ascertainment the genera belonging to the Caryophyllaceae family (USDA Plant Database 2020; Kew Science 2020).
Triterpene saponins
Triterpene saponins constitute the greatest proportion of all phytochemicals known to be present in Caryophyllaceae. The structure of Caryophyllaceae saponins may vary with respect to genera within a family, as well as to plant organs. Oleanane-type saponins, such as gypsogenin, gypsogenic acid, quillaic acid (Fig. 1), 16α-hydroxygypsogenic acid or their derivatives, constitutes the main group of saponins in these plants (Hegnauer 1989; Vincken et al. 2007; Böttger et al. 2011; Cheikh-Ali et al. 2019). For example, this class of compounds is synthesized in Gypsophila altissima (Chen et al. 2010a, b), Gypsophila glomerata (Gevrenova et al. 2018), Gypsophila capillaris (Elgamal et al. 1995), Saponaria officinalis (Koike et al. 1999), Silene vulgaris (Kim et al. 2015), Vaccaria segetalis (Koike et al. 1998), Dianthus versicolor (Ma et al. 2009), Silene cucubalus (Larhsini et al. 2003), Paronychia chionaea (Avunduk et al. 2007) and many other species (Hegnauer 1964; Böttger and Melzig 2011). Moreover, among triterpene saponins from Caryophyllaceae, ursane-type, hopane-type, and lupane-type saponins have also been reported (Vincken et al. 2007). For instance, succulentoside A (Fig. 1) and B, which are hopane-type saponins, were isolated from Polycarpon succulentum (Meselhy and Aboutabl 1997). Gypsophilin (Fig. 1), its glucosyl ester gypsophilinoside and sulfated lupane triterpenes were detected in Gypsophila repens (Elbandy et al. 2007).
Phytoecdysteroids
Phytoecdysteroids, structural analogues of the insect moulting hormone ecdysone, are another group of compounds commonly found in Caryophyllaceae. Several Silene Mill. species, e.g., S. guntensis (Mamadalieva et al. 2011), S. antirrhina, S. chlorifolia, S. cretica, S. disticha, S. echinata, S. italica, S. portensis, S. pseudotites, S. radicosa, S. regia (Meng et al. 2001), S. viridiflora, S. linicola (Mamadalieva et al. 2004), S. nutans, S. otites, and S. tatarica (Bathori et al. 1990), are rich sources of 20-hydroxyecdysone (Fig. 2). Along with 20-hydroxyecdysone, in the genus Silene Mill., a notably large number of structurally various phytoecdysteroids have been observed (Mamadalieva et al. 2014). Furthermore, plants of the genus Coronaria L. are potential producers of ecdysteroid compounds such as viticosterone E, α-ecdysone, taxisterone (Fig. 2), polypodine B, 20,26-dihydroxyecdysone, 2-deoxyecdysterone, and 20-hydroxyecdysone (Mamadalieva et al. 2008). Several ecdysteroids were also established in Silene flos-cuculi (syn. Lychnis flos-cuculi) (Báthori et al. 2001; Dinan et al. 2020). Based on TLC and HPLC analyses, the biotechnological regenerated shoots and roots of L. flos-cuculi, reveals the ability to accumulate 20-hydroxyecdysone and polypodine B (Thiem et al. 2016; Maliński et al. 2019).
Essential oils and volatile compounds
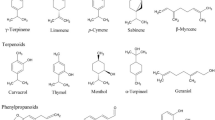
Essential oils are widely distributed in the plant kingdom. This finding suggests that essential oils are also produced in flowering parts of taxa in the pink family. As essential oils are isolated by distillation, they contain a variety of volatile molecules—terpenes and terpenoids, phenol-derived aromatic components, and aliphatic constituents. Components of volatile oils isolated from Dianthus acicularis are chiefly 2-pentadecanone (Fig. 3) and 2-tridecanone, which are presumed to be responsible for the insect repellent activity of this plant (Kirillov et al. 2017). According to analyses of the major constituents of Dianthus calocephalus and Dianthus carmelitarum essential oils, the presence of heneicosane, docosane, tetracosane, phytol, 4,4-dimethyl-2-pentene, pentacosane, and hexahydrofarnesyl acetone (Yücel and Yayli 2018). Additionally, floral fragrance compounds were also established in other Dianthus L. species and Saponaria officinalis with the largest amounts of benzenoids, phenyl propanoids, and isoprenoids (Jürgens et al. 2003). Gas chromatography and gas chromatography combined with mass spectrometry (GLC-MS) examinations of aerial parts of Silene morganae revealed the presence of over 30 compounds with the highest content of monoterpene hydrocarbons being of terpenoids (Azadi and Sohrabi 2014). Furthermore, benzenoids followed by FADs seems to be the dominating compound classes of aromatic compounds in night-blooming or moth-pollinated flowers of Silene Mill. species (Jürgens et al. 2002; Jürgens 2004). Essential oils and their volatile components were also observed in Minuartia recurva (Jovanović et al. 2009), Dianthus caryophyllus (Nerio et al. 2010), Dianthus cruentus (Radulović et al. 2018), some Silene species (Dötterl and Jürgens 2005; Mamadalieva et al. 2014; Mihaylova et al. 2018), Gypsophila bicolor (Shafaghat and Shafaghatlonbar 2011), and two hermaphroditic Schiedea species (Powers et al. 2020).
Sterols
Sterols seem to be useful chemotaxonomic markers at the species level within families of the order Caryophyllales. Atypical for higher plants but predominant in the pink family, the sterol-type class of compounds Δ7-sterols represented by 22-dihydrospinasterol (Fig. 4) occur in Gypsophila perfoliata (Schmidt et al. 1996), Gypsophila paniculata, Silene cucubalus, Arenaria serpyllifolia, Cerastium vulgarum, Cerastium arvense, Myosoton aquaticum, Minuartia caroliniana, Spergula arvensis, Saponaria officinalis, Dianthus armeria, Lychnis alba, Paronychia virginica and Scleranthus annuus (Salt and Adler 1986). Recent research revealed the presence of the α-spinasterol 3-O-β-D-glucoside in the roots of Psammosilene tunicoides (Zhou et al. 2013) and the roots/rhizomes of Silene tatarinowii (Liang et al. 2019).
Cyclic peptides
Cyclic peptides, consisting of a maximum of 14 amino acid residues, are typical N-containing secondary metabolites from Caryophyllaceae (Ma et al. 2006). Genera containing cyclopeptides as major phytochemicals among all plants from this family seem to be Dianthus L., Gypsophila L., Stellaria L., and Vaccaria Mill. For example, the cyclic peptides gypsophins A–F were isolated from the roots of Gypsophila oldhamiana (Wang et al. 2013); the hexapeptides dianthins E, G, and H were found in the aerial parts of Dianthus superbus (Tong et al. 2012); and diandrines A-D (Fig. 5) and drymarins A-B occur in Drymaria diandra (Hsieh et al. 2004a, b; Ding et al. 2000). According to available data, seeds of Vaccaria segetalis are a valuable source of the penta- and hexapeptides segetalin B and segetalin A, respectively (Itokawa et al. 1995; Wang et al. 2011). It is worth mentioning that this group of compounds is present in taxa of the subfamily Alsinoidae, which grow in Antarctica (Jia et al. 2004).
Alkaloids
Another group of nitrogen-containing secondary metabolites are alkaloids, which also occur in Caryophyllaceae to some degree. In particular, alkaloids belonging to the β-carboline group have been described (Dai et al. 2018). For instance, siliendines A–D were isolated from the aerial parts of Silene seoulensis (Seo et al. 2020), drymaritin from the whole plant material of Drymaria diandra (Hsieh et al. 2004a, b), oldhamiaines A and B from the roots of Gypsophila oldhamiana (Zhang et al. 2015), and arenarines A-D from Arenaria kansuensis (Wu et al. 1989; Bracher and Puzik 2004). Phytochemical investigation of the roots of Stellaria dichotoma led to the isolation of 23 various β-carboline-type alkaloids, including stellarines A-B, dichotomides I-XIV, dichotomines A, B, E (Fig. 6), and K, L, glucodichotomine B and 1-acetyl-3-methoxycarbonyl-β-carboline (Chen et al. 2010a, b; Luo et al. 2012). Brachystemma calycinum also produces alkaloids: Brachystemidines A-E were isolated from the roots of this plant (Cheng et al. 2002). Superbusines A and B, which are quinolone alkaloids, were detected in Dianthus superbus (Sun et al. 2019).
Vitamins
Analysis of plant-derived vitamins showed the presence of four tocopherols (α, β, γ, δ) with a different number of methyl substitutions in Silene vulgaris as well as vitamin C and phylloquinone, known as vitamin K1 (Fig. 7). Upon examination of S. vulgaris, the presence of the antioxidant β-carotene, a provitamin of vitamin A, was also reported (Vardavas et al. 2006; Morales et al. 2012; Mamadalieva et al. 2014). Moreover, β-carotene was reported in other Caryophyllaceae plants, e.g., in Stellaria media whose seeds contain vitamin B2 (riboflavin), vitamin B3 (niacin) and vitamin E (Slavokhotova et al. 2011; Taskin and Bitis 2013).
Phenolic compounds
Phenolic compounds constitute a large proportion of secondary metabolites in Caryophyllaceae plants. Phenolic acids are the main polyphenols produced by plants. However, only a few publications report on phenolic compound isolation and identification in Caryophyllaceae. For instance, caffeic acid was obtained from aerial parts of Silene (syn. Lychnis) flos-cuculi (Tomczyk 2008), p-coumaric acid, dihydroferulic acid, and syringic acid were identified in the ground roots, stems, leaves, and flowers of Gypsophila paniculata (Chou et al. 2008); and Dianthus species are a source of gentisic acid, a commonly reported aromatic acid in green plants (Griffiths 1959). Fractionation of a Gypsophila sphaerocephala extract resulted in the isolation of 3,4-dihydroxybenzoic acid, syringic acid, p-hydroxybenzoic acid, and rosmarinic acid (Fig. 8) from the methanol extract and rosmarinic acid and syringic acid from the water extract (Altay et al. 2018). Additionally, the Silene Mill. genus is also known as a source of phenolic acids (Mamadalieva et al. 2014). Derivatives of cinnamic acid or benzoic acid and aromatic amino acids (anthranilic acid), so-called anthranilamides with phytoalexin-related activity, are commonly found in parts of Dianthus caryophyllus infected by pathogens (Niemann 1993).
Catechins (flavanol derivatives) are similar in structure to flavonols, except for the lack of a carbonyl group in the pyran ring (Heim et al. 2002). Plants of the Caryophyllaceae family were also screened for flavanols, but only a few species, including Herniaria fontanessii (Mbark et al. 1999) and Arenaria kansuensis (Liu et al. 2018), contained this group of compounds. The major flavanols, catechin, and epicatechin, act as strong antioxidant agents similar to other polyphenols (Iacopini et al. 2008).
Lignans, insoluble elements of certain cell walls, are rather uncommon phytochemicals in Caryophyllaceae, except for Pteranthus dichotomus, which contains 8-oxo-pinoresinol (Allaoua et al. 2016).
Unlike the many taxa of the order Caryophyllales that produce betalains as coloured flower pigments, Caryophyllaceae produce anthocyanins: cyanidin glycoside derivatives were identified in Silene dioica (Kamsteeg et al. 1976; Kamsreeo et al. 1980) and S. armeria (Mamadalieva et al. 2014). Cyclic malyl anthocyanins were isolated from deep pink and red–purple Dianthus caryophyllus flower petals (Nakayama et al. 2000). Moreover, the genus Lychnis is a source of the anthocyanin aglycones named anthocyanidins, such as cyanidin, peonidin, and pelargonidin (Fig. 9), as well as their glycoside derivatives (Kuwayama et al. 2005).
Among the many polyphenolic phytoconstituents occurring in this family, tannins are also present and have physiological activity against herbivores. Tannins were detected in some Minuartia species (Zaychenko and Zernov 2017), Stellaria laeta (Jung et al. 1979), Polycarpaea corymbosa (Balamurugan et al. 2013), Drymaria cordata (Baruah et al. 2009), Silene vulgaris (Kim et al. 2015), Silene compacta (Bakroglu et al. 2014), and Spergula fallax (Aldhebiani and Mufarah 2017).
However, flavonoid compounds remain one of the most extensive groups of polyphenols in Caryophyllaceae, and novel compounds are yet to be identified. The aglycones and their glycosides are probably present in almost all plants.
Flavonoids of the Caryophyllaceae and their main biological activities
Flavonoids are low-molecular-weight secondary plant metabolites composed of two benzene rings and one heterocyclic pyran ring that are chemically divided into groups according to their chemical substitutions. Flavonoid moieties can be modified by glycosylation, hydrogenation, hydroxylation, and methylation as well as malonylation and sulfatation. The chemical and biological activities of flavonoids and their derivatives are connected with their structure and the position of various substitutions on the molecule. The general activity of polyphenols concerns the reactivity of their phenolic OH groups. The hydroxyl groups can dissociate under physiological conditions to negatively charged phenolate ions. Thus, polyphenols can interact with proteins by forming hydrogen bridges and, more importantly, ionic bonds with positively charged amino groups. As a consequence, the bioactivity of proteins can be directly changed when a polyphenol binds to a receptor side or active centre of an enzyme. Polyphenols, especially those with several phenolic OH groups (such as rosmarinic acid or tannins), can change the 3D structure of proteins and impair their bioactivity. Because of these interactions, polyphenols affect many proteins in the human body and in microbes that are medicinally relevant. This is the mechanism by which plant polyphenols are medicinally active (Wink 2015; van Wyk and Wink 2017).
The biological activities of flavonoids may be also connected with their metabolites, which are produced in vivo. The gastrointestinal tract reveals primary role in the absorption, distribution, metabolism and excretion of flavonoids, which are substrates for conjugating and hydrolyzing enzymes in the small intestine, liver, and colon to O-glucuronides, O-methyl and sulfate esters. Firstly, metabolism of flavonoids take place in the small intestine followed by the liver where they are transformed and then produced glucuronides and sulfate derivatives. Flavonoid compounds that reach the colon are catabolize to low molecular weight phenolic acids by the intestinal microflora (Thilakarathna and Rupasinghe 2013). An anaerobic bacteria found in human gastrointestinal tract (e.g. Eubacterium ramulus) splits the ring structures of several flavonols and flavones leading to the formation of aglycones and common phenolics intermediates consisting of hydroxyphenylacetic, hydroxyphenylpropionic, acetate, and butyrate acids with varying degrees of hydroxylation (Blaut et al. 2003; Serra et al. 2012; Pei et al. 2020). The amount of urinary excretion demonstrates that the colonic catabolites are absorbed into the portal vein and this way run over the body in the circulatory system (Crozier et al. 2010). The flavonoid glucuronides and sulfate derivatives facilitate their excretion through urine and bile (Thilakarathna and Rupasinghe 2013). Urinary excretion of < 1.0% confirms that C-flavones are poorly absorbed, and 10–88% recovery from feces indicates that they may be resistant to degradation by gut bacteria in rats (Ma et al. 2010). As with flavone O-glycosides, the C-glycosides are less bioavailable in humans than in rats. Nevertheless, it is known that the absorption of dietary flavonoids may be affected by the food matrix, the metabolic processes mediated by the liver, intestine, kidneys, as well as colon microbiota (Hollman 2004; Viskupičová et al. 2008; Hostetler et al. 2017; Cosme et al. 2020; Di Lorenzo et al. 2021).
To the best of our knowledge, apigenin, found in 28 species, is the major flavone in Caryophyllaceae plants. The apigenin exhibits cancer chemopreventive activity such as antiproliferative effects on human breast cancer cells, inhibition of cell growth by apoptosis in cervical carcinoma, or selective apoptotic effects in monocytic and lymphocytic leukaemias (Shukla and Gupta 2010; Imran et al. 2020). A similar number of species contain another widely distributed aglycone—luteolin. As with many other polyphenols, luteolin is a powerful antioxidant that can prevent inflammation and allergies and suppress the expression of cancer-promoting proteins (Imran et al. 2019a, b). Other important flavones are the luteolin 8-C-glucoside and apigenin 8-C-glucoside, orientin and vitexin, respectively. Plants rich in orientin are often used in traditional medicine for the treatment of respiratory disorders, pharyngitis, skin disorders, common cold, and mild anxiety (Grundmann et al. 2008; Lam et al. 2016). In addition, luteolin 8-C-glucoside acts as an antioxidant, antiaging, anti-inflammatory, cardioprotective, radioprotective, and neuroprotective agent (Uma Devi et al. 2000; Praveena et al. 2014; Lam et al. 2016). Vitexin, successfully isolated from Caryophyllaceae, exhibits various medicinal properties, such as fat reduction, improved glucose metabolism, hepatoprotection, neuroprotection, cardioprotection, and even anticancer activity (Ganesan and Xu 2017; Peng et al. 2020).
Kaempferol exhibits multiple biological effects, such as antioxidant, anti-inflammatory, antidiabetic, antiaging, and antimicrobial effects, and is being applied in the chemotherapy of skin, liver, and colon tumours (Zhu et al. 2018; Cho and Park 2013; Imran et al. 2019a, b). Furthermore, kaempferol can be used in the treatment of cardiovascular diseases, degenerative disorders, diabetes, and microbial contamination diseases (Imran et al. 2018, 2019a). The flavonol aglycone, quercetin can function as an antioxidant as well as a blood pressure-lowering and anticancer agent (Kukongviriyapan et al. 2012; Egert et al. 2009). Moreover, quercetin can decrease the levels of proinflammatory cytokines, e.g., interleukin 6, 8, 1β, and TNFα (Wang et al. 2016). Rutin, a 3-O-rutinoside derivative of quercetin established in 18 different species of the Caryophyllaceae, is also often used in studies due to its extensive therapeutic properties: The health-promoting effects of rutin are linked with antioxidant, cytoprotective, neuroprotective, vasoprotective, and cardioprotective activities (Kim et al. 2009; Ganeshpurkar and Saluja 2017). The results from some studies also indicated a positive effect of rutin on Parkinson's and Alzheimer's diseases (Gullón et al. 2017). The main strategies for a neurodegenerative disease therapy involves the reduction of reactive oxygen species and amyloid beta-protein production, and the activation of mechanisms of neuronal death (de Andrade Teles et al. 2018).
The main biological activities of hesperidin isolated from Herniaria hemistemon (Elhagali et al. 2019) are chemotherapeutic, antiallergic, anti-inflammatory, endocrine, cardiovascular, and organ-protective effects (Kumar et al. 2008; Zanotti et al. 2013; Ganeshpurkar and Saluja 2019). The aglycone naringenin exhibited multiple therapeutic effects associated with its free radical-scavenging properties. Depending on the concentration and method of administration, naringenin can be useful in the treatment of viral, bacterial, and inflammatory diseases and obesity (Ke et al. 2016; Kozlowska et al. 2017; Salehi et al. 2019). In addition, naringenin was tested for its potential anticancer activity and as a cardioprotective agent (Salehi et al. 2019). A wide range of therapeutic properties of naringin, a 7-hesperidoside derivative of naringenin, include the treatment of metabolic syndrome, oxidative stress, and conditions of the central nervous system (Sachdeva et al. 2014; Dhanya et al. 2015; Chen et al. 2016).
A medically useful group of flavonoids are isoflavones, which are also known as phytoestrogens (Heim et al. 2002). These compounds can bind to receptors of oestrogen and oestrogen hormone binding protein and inhibit an important enzyme of angiogenesis and tumour formation, tyrosinase (Wink 2015). It was concluded that plants rich in isoflavones are effective in treating cardiovascular and osteoporosis disorders as well as in reducing postmenopausal symptoms (Clarkson 2002; Atkinson et al. 2004; Vitale et al. 2013). To date, the distribution of genistein and daidzein is common in several legumes of the Fabaceae family, such as soybean (Bustamante-Rangel et al. 2018). However, there are reports of the presence of genistein in a species of the Caryophyllaceae family, e.g., Stellaria dichotoma or Stellaria holostea (Mikšátková et al. 2014).
Our approach included screening for flavonoid aglycones and their highly glycosylated derivatives within Caryophyllaceae family (Cook and Samman 1996). The flavonoid aglycone and glycoside group remains one of the most extensive groups of polyphenols in Caryophyllaceae. Most of these compounds occur in Silene L., Dianthus L. (Obmann et al. 2011a, b; Boguslavskaya et al. 1983), Gypsophila L. (Zhang et al. 2011a, b; Zheleva-Dimitrova et al. 2018), Stellaria L. (Mikšátková et al. 2014), Spergularia Presl. (Ferreres et al. 2011) and Herniaria L. (Elhagali et al. 2019; El Mabruki et al. 2014). Nevertheless, flavonoid compounds are probably present in almost all plants. We assembled information regarding their presence in 26 genera and over 120 species of the Caryophyllaceae family (see Table 1).
Flavones
One of the most pharmacologically valuable flavonoid classes present is that comprising flavones, which can be synthesized by various pathways, depending on whether they contain C- or O-glycosylation, O-methylation acylation, and hydroxylated B-ring. These compounds undergo characteristic reactions ascribed to three functional structures—hydroxyl and carbonyl groups and a double bond (Singh et al. 2014; Panche et al. 2016). Their natural distribution is demonstrated for almost all plant tissues (Figs. 10, 11, 12).
Flavonols
An additional class of flavonoids commonly found in Caryophyllaceae is that comprising flavonols, including kaempferol, quercetin and its glycoside rutin (quercetin 3-O-rutinoside). Flavonols, compared to flavones, carry an additional hydroxyl group in the pyran ring (Panche et al. 2016) (Fig. 13).
Flavonones (dihydroflavones)
Flavonones (dihydroflavones) differ from flavones by the lack of a double bond in the pyran ring. Hesperidin, naringenin, and its glycoside naringin (naringenin 7-hesperidoside) are commonly found in citrus fruits (Panche et al. 2016), but they can also be found in certain species of the pink family.
Isoflavones
Phytoestrogens are non-steroidal polyphenolic compounds occurring in plants and can be chemically divided into two main groups: flavonoids (isoflavones) and non-flavonoids (lignans). The structure of isoflavone aglycone consists of a 3‐phenylchroman ring that is substituted with hydroxyl groups in the positions C4′ and C7 (Bustamante-Rangel et al. 2018; Krížová et al. 2019) (Fig. 14).
Because flavonoids are widely distributed in the plant kingdom and their presence in Caryophyllaceae plants has not been published until now, the authors of the article summarized the phytochemistry of 26 flavonoid-producing genera and relevant species. The flavonoid compounds occurring in Caryophyllaceae, the corresponding species and the literature references are summarized in Table 1.
Conclusions
The Caryophyllaceae family contains a large number of genera and species that are widely distributed over different climate zones. It is evident that the plants from this family produce a wide range of pharmaceutically promising, interesting, and valuable flavonoids and other secondary metabolites. Phytochemical data of flavonoids in plants of this family have not been published until now. Despite the dominant proportion of triterpene saponins among all phytoconstituents, polyphenols, including flavonoid compounds, remain a large group of compounds with health-related activity, such as antioxidant, anti-inflammatory, antimicrobial, organ-protective, and even anticancer effects (van Wyk and Wink 2017; Imran et al. 2019a; Ganeshpurkar and Saluja 2019). Our approach involved screening flavonoid-containing species, including those containing aglycones and their glycoside derivatives, which could be identified in 26 genera and more than 120 species within the Caryophyllaceae.
To the best of our knowledge, apigenin is the most common aglycone in this family and can be found in 28 different species, such as Vaccaria segetalis (Baeva et al. 1975), Stellaria media (Melnyk et al. 2018), Silene saxatilis (Zemtsova et al. 1975), Pteranthus dichotomus (Allaoua et al. 2016), Silene (Lychnis) flos-cuculi (Tomczyk 2008), Herniaria glabra (El Mabruki et al. 2014) and others. Furthermore, the C-bonded apigenin glucoside isovitexin has been isolated from more than 70 plants, making it the predominant flavonoid within this family. On the basis of the data collected in Table 1, it was concluded that the highly glycosylated C- and O-flavonoids (apigenin, luteolin, chrysoeriol, kaempferol, quercetin, formononetin, genistein, myricetin, tectorigenin) with either one, two or three sugar moieties, as presented in this review, are commonly found in the Caryophyllaceae family. The genera Silene Mill., Dianthus L., Stellaria L., Herniaria L., Spergularia Presl., Gypsophila L. and Cerastium L. appear to contain high abundances of flavonoid compounds.
In summary, the structural diversity of flavonoids established in the Caryophyllaceae family makes them an interesting object of phytochemical and pharmacological investigations.
References
Adjadj M, Baghiani A, Boumerfeg S, Noureddine C, Khennouf S, Arrar L, Mubarak MS (2015) Protective effect of Paronychia argentea L. on acetic acid induced ulcerative colitis in mice by regulating antioxidant parameters and inflammatory markers. Wulfenia J 22:148–172
Ahmad V, Ali Z, Ali M, Zahid M (1998) Chemical constituents of Silene conoidea. Fitoterapia 69:406–408
Aldhebiani AY, Mufarah N (2017) Phytochemical screening of some wild plants from Wadi Yalmlam, Saudi Arabia. IOSR J Pharm Biol Sci 12:25–27
Ali Z, Ahmad VU, Ali MS, Iqbal F, Zahid M, Alam N (1999) Two new C-glycosylflavones from Silene conoidea. Nat Prod Lett 13:121–129
Allaoua Z, Benkhaled M, Dibi A, Long C, Aberkane MC, Bouzidi S, Haba H (2016) Chemical composition, antioxidant and antibacterial properties of Pteranthus dichotomus from Algerian Sahara. Nat Prod Res 30:700–704
Al-Snafi AE (2017) Chemical contents and medical importance of Dianthus caryophyllus—a review. ISOR J Pharm 7:61–71
Altay A (2018) HPLC Analysis of phenolic compounds from Gypsophila aucheri Boiss. and investigation of antioxidant and cytotoxic activity of Gypsophila aucheri Boiss. extracts. J Sci Technol 11:168–181
Altay A, Degirmenci S, Korkmaz M, Cankaya M, Koksal E (2018) In vitro evaluation of antioxidant and anti-proliferative activities of Gypsophila sphaerocephala (Caryophyllaceae) extracts together with their phenolic profiles. J Food Meas Charact 12:2936–2945
Altay A, Tohma H, Durmaz L, Taslimi P, Korkmaz M, Gulcin I, Koksal E (2019) Preliminary phytochemical analysis and evaluation of in vitro antioxidant, antiproliferative, antidiabetic, and anticholinergics effects of endemic Gypsophila taxa from Turkey. J Food Biochem 43:1–11
Amosova EN, Zueva EP, Lopatina KA, Safonova EA, Razina TG, Rybalkina OY, Zibareva LN (2019) Influence of Lychnis chalcedonica L. flavonoids on transplanted tumor development and cytostatic therapy effectiveness in mice. Pharm Chem J 53:454–457
Ancheeva E, Daletos G, Muharini R, Lin WH, Teslov L, Proksch P (2015) Flavonoids from Stellaria nemorum and Stellaria holostea. Nat Prod Commun 10:437–440
Atkinson C, Compston JE, Day NE, Dowsett M, Bingham SA (2004) The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 79:326–333
Atta EM, Nassar AA, Hasan NM, Hasa AR (2013) New flavonoid glycoside and pharmacological activities of Pteranthus dichotomus forssk. Rec Nat Prod 7:69–79
Avunduk S, Lacaille-Dubois MA, Miyamoto T, Bedir E, Şenol SG, Çalişkan ÖA (2007) Chionaeosides A-D, triterpene saponins from Paronychia chionaea. J Nat Prod 70:1830–1833
Azadi B, Sohrabi Y (2014) Chemical composition of Silene morganae Freyn volatile oil. Nat Prod Res 29:791–794
Baeva RT, Karryev MO, Litvinenko VI, Abubacirov NK (1975) Glycosides of Vaccaria segetalis V. Vaccarin. Chem Nat Compd 10:182–186
Bahar A, Mubashir HM, Shamshir K (2008) Lychnis coronaria Linn. A review. Nat Prod Indian J 4:22–25
Bakroglu A, Kökten K, Kavurmaci Z (2014) Tannin, protein contents and fatty acid compositions of Silene compacta Fische seeds from Bingöl, Turkey. Turk J Agric Nat Sci 1:441–444
Balamurugan K, Sakthidevi G, Mohan VR (2013) Antiulcer activity of Polycarpaea corymbosa (L.) Lam. whole plant extracts (Caryophyllaceae). Int J Biol Med Res 4:3379–3382
Balsevich JJ, Ramirez-Erosa I, Hickie RA, Dunlop DM, Bishop GG, Deibert LK (2011) Antiproliferative activity of Saponaria vaccaria constituents and related compounds. Fitoterapia 83:170–181
Baruah CC, Pal SK, Baruah AG, Roy JD, Buragohain B, Bora RS, Lahon LC (2009) Analgesic activity of methanolic extract of Drymaria cordata Willd Caryophyllaceae. Pharmacologyonline 2:470–476
Bathori M, Varga E, Szendrei K, Lafont R (1990) Isolation and identification of new edcysteroids from the Caryophyllaceae. J Nat Prod 5:279–293
Báthori M, Lafont R, Girault JP, Máthé I (2001) Structural diversity of ecdysteroids of Lychnis flos-cuculi. Acta Pharm Hung 71:157–167
Bechlem H, Mencherini T, Bouheroum M, Benayache S, Cotugno R, Braca A, De Tommasi N (2017) New constituents from Gymnocarpos decander. Planta Med 83:1200–1206
Blaut M, Schoefer L, Braune A (2003) Transformation of flavonoids by intestinal microorganisms. Int J Vitam Nutr Res 73:79–87
Boguslavskaya LI (1976) Phenolic compounds of Dianthus platycodon. Chem Nat Compd 12:485
Boguslavskaya LI, Dem’yanenko SI, Salam DK (1983) Flavonoids of some species of the genus Dianthus. Khimiya Prirodnykh Soedineni 61:366
Boguslavskaya LI, Tikhonov AI, Pashnev PD, Zhemal B, Sklyar VI (1985a) C-glycosides of Stellaria holostea. Khim Prir Soedin 21:385
Boguslavskaya LI, Tikhonov AI, Pashnev PD, Jemal B, Sklyar VI (1985b) Flavonoid compounds of Herniaria polygama. Chem Nat Compd 21:386–411
Böttger S, Melzig MF (2011) Triterpenoid saponins of the Caryophyllaceae and Illecebraceae family. Phytochem Lett 4:59–68
Bouillant ML, de Arce FF, Favre-Bonvin J, Chopin J, Zoll A, Mathieu G (1979) Nouvelles C-glycosylflavones extraites de Spergularia rubra. Phytochemistry 18:1043–1047
Braca A, Bader A, Siciliano T, De Tommasi N (2008) Secondary metabolites from Paronychia argentea. Magn Reson Chem 46:88–93
Bracher F, Puzik A (2004) β-Carboline Alkaloids 9 [1]. Total synthesis of the β-carboline alkaloids arenarine A and (±)-arenarine B. J Heterocycl Chem 41:173–176
Brahmachari G, Gorai D (2006) Progress in the research on naturally occurring flavones and flavonols: an overview. Curr Org Chem 10:873–898
Brockington SF, Walker RH, Glover BJ, Soltis PS, Soltis DE (2011) Complex pigment evolution in the Caryophyllales. New Phytol 190:854–864
Bustamante-Rangel M, Delgado-Zamarreňo MM, Pèrez-Martin L, Rodriguez-Gonzalo E, Dominguez-Alvarez J (2018) Analysis of isoflavones in foods. Compr Rev Food Sci Food Saf 17:391–411
Cambie RC (1959) Identity of isovitexin (“homovitexin”) and saponaretin. Chem Ind 1959:87–88
Cheikh-Ali S, Farman M, Lacaille-Dubois MA, Semmar N (2019) Structural organization of saponins in Caryophyllaceae. Phytochem Rev 18:405–441
Chen Q, Luo JG, Kong LY (2010a) Triterpenoid saponins from Gypsophila altissima L. Chem Pharm Bull 58:412–414
Chen YF, Kuo PC, Chan HH, Kuo IJ, Lin FW, Su CR, Wu TS (2010b) β-carboline alkaloids from Stellaria dichotoma var. lanceolata and their anti-inflammatory activity. J Nat Prod 73:1993–1998
Chen R, Qi QL, Wang MT, Li QY (2016) Therapeutic potential of naringin: an overview. Pharm Biol 54:3203–3210
Cheng YX, Zhou J, Tan NH, Teng RW, Lu Y, Wang C, Zheng QT (2002) Isolation and characterization of brachystemidines A-E, novel alkaloids from Brachystemma calycinum. J Nat Prod 65:750–752
Cheriti A, Sekkoum K (1996) Flavonoids from Herniaria mauritanica. Indian J Pharm Sci 58:203–204
Cho HJ, Park JHY (2013) Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J Cancer Prev 18:257–263
Cho JY, Kim MS, Lee YG, Jeong HY, Lee HJ, Ham KS, Moon JH (2016) A phenyl lipid alkaloid and flavone C-diglucosides from Spergularia marina. Food Sci Biotechnol 25:63–69
Chopin MJ, Bouillant ML, Wagner H, Galle K (1974) Endgültige struktur von schaftosid aus Silene schafta. Phytochemistry 13:2583–2586
Chou S, Everngam MC, Beck JJ (2008) Allelochemical phenolic acids from Gypsophila paniculata. J Undergraduate Chem Res 7:2–4
Clarkson TB (2002) Soy, soy phytoestrogens and cardiovascular disease. J Nutr 132:566S-569S
Cook NC, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. Nutritional Biochemistry 7:66–76
Cosme P, Rodríguez AB, Espino J, Garrido M (2020) Plant phenolics: bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 9:1–20
Crozier A, Del Rio D, Clifford MN (2010) Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med 31:446–467
Cui YL, Shen N, Zhao JQ, Dang J (2017a) Phytochemical constituents of Arenaria kansuensis. Chem Nat Compd 53:1002–1004
Cui Y, Shen N, Yuan X, Dang J, Shao Y, Mei L, Liu Z (2017b) Two-dimensional chromatography based on on-line HPLC-DPPH bioactivity-guided assay for the preparative isolation of analogue antioxidant compound from Arenaria kansuensis. J Chromatogr B 1046:81–86
Cui Y, Tao Y, Wang S (2018) Antihypoxic activities of constituents from Arenaria kansuensis. Phytomedicine 38:175–182
Cui Y, Shao Y, Wang Q, Mei L, Tao Y (2019) Purification of flavonolignan diastereoisomers from Arenaria kansuensis by two-dimensional liquid chromatography combined with solid-phase extraction. J Chromatogr Sci 57:1–8
Curir P, Dolci M, Lanzotti V, Taglialatela-Scafati O (2001) Kaempferide triglycoside: A possible factor of resistance of carnation (Dianthus caryophyllus) to Fusarium oxysporum f. sp. dianthi. Phytochemistry 56:717–721
Curir P, Lanzotti V, Dolci M, Dolci P, Pasini C, Tollin G (2003) Purification and properties of a new S-adenosyl-L-methionine: flavonoid 4′-O-methyltransferase from carnation (Dianthus caryophyllus L.). Eur J Biochem 270:3422–3431
Curir P, Dolci M, Galeotti F (2005) A phytoalexin-like flavonol involved in the carnation (Dianthus caryophyllus)—Fusarium oxysporum f. sp. dianthi pathosystem. J Phytopathol 153:65–67
Dai J, Dan W, Schneider U, Wang J (2018) β-Carboline alkaloid monomers and dimers: occurrence, structural diversity, and biological activities. Eur J Med Chem 157:622–656
Darmograi VN (1977) Flavonoids of plants of the genera Silene and Otites adans, family Caryophyllaceae. Chem Nat Compd 13:102–103
Darmograi VN (1979) Flavonoids of some species of the genera Arenaria and Cerastium. Khim Prir Soedin 1:93255
de Andrade Teles RB, Diniz TC, Pinto TCC, de Oliveira Júnior RG, Silva MG, de Lavor EM, Fernandes AWC, de Oliveira AP, de Almeida Ribeiro FPR, da Silva AAM, Cavalcante TCF, Quintans Júnior LJ, da Silva Almeida JRG (2018) Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev 21:1–21
del Valle JC, Buide ML, Casimiro-Soriguer I, Whittall JB, Narbona E (2015) On flavonoid accumulation in different plant parts: Variation patterns among individuals and populations in the shore campion (Silene littorea). Front Plant Sci 6:1–13
Devkota HP, Fukusako K, Ishiguro K, Yahara S (2013) Flavone C-glycosides from Lychnis senno and their antioxidative activity. Nat Prod Commun 8:1413–1414
Dhanya R, Arun KB, Nisha VM, Syama HP, Nisha P, Santhosh Kumar TR, Jayamurthy P (2015) Preconditioning L6 muscle cells with naringin amelio-rates oxidative stress and increases glucose uptake. PLoS ONE 10:e0132429
Di Lorenzo Ch, Colombo F, Biella S, Stockley C, Restani P (2021) Polyphenols and human health: the role of bioavailability. Nutriens 13:1–30
Dinan L, Balducci C, Guibout L, Lafont R (2020) Small-scale analysis of phytoecdysteroids in seeds by HPLCDAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem Anal 31:1–19
Ding Z, Zhou J, Tan N (1999) A novel flavonoid glycoside from Drymaria diandra. Planta Med 65:578–579
Ding Z, Zhou J, Tan N, Teng R (2000) Two new cyclic peptides from Drymaria diandra. Planta Med 66:386–388
Ding ZT, Yang XQ, Cao QE, Li F (2005) Three new flavone glycosides from Drymaria diandra Bl. J Integr Plant Biol 47:1140–1144
Dong Q, Huang Y, Qiao S (2007) Studies on chemical constituents from Stellaria media. Chin Mater Med 32:1048–1051
Dötterl S, Jürgens A (2005) Spatial fragrance patterns in flowers of Silene latifolia: Lilac compounds as olfactory nectar guides? Plant Syst Evol 255:99–109
Dubois M, Zoll A, Bouillant M, Delaveau P (1982) Di-C-Glycosylflavones du Cerastium arvense ssp. arvense nouvelles pour les Caryophyllaceae. Planta Med 46:56–57
Dubois MA, Zoll A, Chopin J (1983) 7,2″-di-O-Glycosyl-6-C-glycosylflavones from Cerastium arvense. Phytochemistry 22:2879–2880
Dubois MA, Zoll A, Markham KR, Bouillant ML, Dellamonica G, Chopin J (1984) 6-C-β-D-glucopyranosyl-8-C-β-D-galactopyranosylapigenin from Cerastium arvense. Phytochemistry 23:706–707
Dubois MA, Zoll A, Chopin J (1985) Isomollupentin-O-glucosides from Cerastium arvense. Phytochemistry 24:1077–1080
Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, Wolffram S, Müller MJ (2009) Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 7:1065–1074
El Mabruki K, Klemper AV, Kaukhova IE, Sorokin VV (2014) Establishment of rupturewort (Herniaria glabra) herb identity characterestics and quality indices. Pharmacia 6:21–24
Elbandy M, Miyamoto T, Lacaille-Dubois MA (2007) Sulfated lupane triterpene derivatives and a flavone C-glycoside from Gypsophila repens. Chem Pharm Bull 55:808–811
El-Dien OG, Shawky E, Aly AH, Abdallah RM, Abdel-Salam NA (2013) A validated high-performance thin-layer chromatography (HPTLC) method for the quantitative determination of tricin in two Spergularia Species. Am J Anal Chem 4:668–673
Elgamal MHA, Soliman HSM, Karawya MS, Mikhova B, Duddeck H (1995) Isolation of triterpene saponins from Gypsophila capillaris. Phytochemistry 38:1481–1485
Elhagali G, Abozeed A, Abdelnaser K, Youssif Y (2019) Investigation of bioactive constituents and biological activities of different fractions from Herniaria hemistemon. J Gay Al-Azhar Bull Sci 30:67–80
El-Hawary SS, Mubarek MM, Lotfy AR, Hassan AR, Sobeh M, Okba MM (2020) Validation of antidiabetic potential of Gymnocarpos decandrus Forssk. Nat Prod Res 13:1–6
Ferreres F, Gil-Izquierdo A, Vinholes J, Grosso C, Valentão P, Andrade PB (2011) Approach to the study of C-glycosyl flavones acylated with aliphatic and aromatic acids from Spergularia rubra by high-performance liquid chromatography-photodiode array detection/electrospray ionization multi-stage mass spectrometry. Rapid Commun Mass Spectrom 25:700–712
Fukui Y, Tanaka Y, Kusumi T, Iwashita T, Nomoto K (2003) A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 63:15–23
Galeotti F, Barile E, Curir P, Dolci M, Lanzotti V (2008a) Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem Lett 1:44–48
Galeotti F, Barile E, Lanzotti V, Dolci M, Curir P (2008b) Quantification of major flavonoids in carnation tissues (Dianthus caryophyllus) as a tool for cultivar discrimination. Z Naturforsch 63C:161–168
Ganesan K, Xu BJ (2017) Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Ann N Y Acad Sci 1401:102–113
Ganeshpurkar A, Saluja AK (2017) The pharmacological potential of rutin. Saudi Pharm J 25:149–164
Ganeshpurkar A, Saluja A (2019) The pharmacological potential of hesperidin. Indian J Biochem Biophys 56:287–300
Gevrenova R, Bardarov K, Bouguet-Bonnet S, Voynikov Y, Balabanova V, Zheleva-Dimitrova D, Henry M (2018) A new liquid chromatography-high resolution orbitrap mass spectrometry-based strategy to characterize glucuronide oleanane-type triterpenoid carboxylic acid 3, 28-O-bidesmosides (GOTCAB) saponins. A case study of Gypsophila glomerata Pall ex M. B. (Caryophyllaceae). J Pharm Biomed Anal 159:567–581
Griffiths LA (1959) On the distribution of gentisic acid in green plants. J Exp Bot 10:437–442
Grundmann O, Wang J, McGregor GP, Butterweck V (2008) Anxiolytic activity of a phytochemically characterized Passiflora incarnata extract is mediated via the GABAergic system. Planta Med 74:1769–1773
Gullón B, Lú-Chau TA, Moreira MT, Lema JM, Eibes G (2017) Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci Technol 67:220–235
Hegnauer R (1964) Chemotaxonomy of plants, vol 18. Springer Basel AG, Basel, p 379
Hegnauer R (1989) Caryophyllaceae. Chemotaxonomy of Plants, vol 30. Springer Basel AG, Basel, pp 215–220
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Heinsbroek R, van Brederode J, van Nigtevecht G, Maas J, Kamsteeg J, Besson E, Chopin J (1980) The 2″-O-glucosylation of vitexin and isovitexin in petals of Silene alba is catalysed by two different enzymes. Phytochemistry 19:1935–1937
Hollman PCH (2004) Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol 42:74–83
Hostetler GL, Ralston RA, Schwartz SJ (2017) Flavones: food sources, bioavailability, metabolism, and bioactivity. Adv Nutr 28:423–435
Hsieh PW, Chang FR, Lee KH, Hwang TL, Chang SM, Wu YC (2004a) A new anti-HIV alkaloid, drymaritin, and a new C-glycoside flavonoid, diandraflavone, from Drymaria diandra. J Nat Prod 67:1175–1177
Hsieh PW, Chang FR, Wu CC, Wu KY, Li CM, Wang WY, Wu YC (2004b) Selective inhibition of collagen-induced platelet aggregation by a cyclic peptide from Drymaria diandra. Helv Chim Acta 87:57–66
Huang QF, Zhang SJ, Zheng L, Liao M, He M, Huang RB, Lin X (2012) Protective effect of isoorientin-2’-O-α-L-arabinopyranosyl isolated from Gypsophila elegans on alcohol induced hepatic fibrosis in rats. Food Chem Toxicol 50:1992–2001
Hussein IA, Srivedavyasasri R, El-Hela AA, Mohammad AI, Ross SA (2019) Antimicrobial secondary metabolites from Silene rubella growing in Egypt. J Biomed Pharm Res 8:81–84
Hussein IA, Srivedavyasasri R, El-Hela AA, Mohammad AI, Ross SA (2020) Chemical constituents from Silene schimperiana Boiss. belonging to Caryophyllaceae and their chemotaxonomic significance. Biochem Syst Ecol 92:1–4
Iacopini P, Baldi M, Storchi P, Sebastiani L (2008) Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: content, in vitro antioxidant activity and interactions. J Food Compos Anal 21:589–598
Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, Mubarak MS (2018) Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res 2018:1–13
Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Mubarak MS (2019a) Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother 112:108612
Imran M, Salehi B, Sharifi-rad J, Gondal TA, Saeed F, Imran A, Estevinho LM (2019b) Kaempferol: A key emphasis to its anicancer potential. Molecules 24:1–16
Imran M, Aslam GT, Atif M, Shahbaz M, Batool QT, Hanif MM, Sharifi-Rad J (2020) Apigenin as an anticancer agent. Phytother Res 26:1–17
Itokawa H, Yun Y, Morita H, Takeya K, Yamada K (1995) Estrogen-like activity of cyclic peptides from Vaccaria segetalis extracts. Planta Med 61:561–562
Iwashina T, Yamaguchi MA, Nakayama M, Onozaki T, Yoshida H, Kawanobu S, Okamura M (2010) Kaempferol glycosides in the flowers of carnation and their contribution to the creamy white flower color. Nat Prod Commun 5:1903–1906
Jakimiuk K, Strawa JW, Granica S, Tomczyk M (2020) Flavonoids from the aerial parts of Scleranthus perennis. T20 PSE Conference Liverpool 2020, "Contemporary Natural Products Discovery Research", 6.03.2020, Liverpool, United Kingdom, p. 57
Jia AQ, Tan NH, Yang YP, Wu SG, Wang LQ, Zhou J (2004) Cyclopeptides from three arctic Caryophyllaceae plants, chemotaxonomy and distribution significance of Caryophyllaceae cyclopeptides. Acta Bot Sin 46:625–630
Jovanović O, Radulović N, Palić R, Zlatković B (2009) Volatiles of Minuartia recurva (All.) Schinz et Thell. subsp. recurva (Caryophyllaceae) from Serbia. J Essent Oil Res 21:429–432
Jung HJG, Batzli GO, Seigler DS (1979) Patterns in the phytochemistry of arctic plants. Biochem Syst Ecol 7:203–209
Jürgens A (2004) Flower scent composition in diurnal Silene species (Caryophyllaceae): phylogenetic constraints or adaption to flower visitors? Biochem Syst Ecol 32:841–859
Jürgens A, Witt T, Gottsberger G (2002) Flower scent composition in night-flowering Silene species (Caryophyllaceae). Biochem Syst Ecol 30:383–397
Jürgens A, Witt T, Gottsberger G (2003) Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem Syst Ecol 31:345–357
Kamsreeo J, van Brederode J, van Nigtevecht G (1980) Genetical and biochemical evidence that the hydroxylation pattern of the anthocyanin B-ring Silene dioica is determined at the p-coumaroyl-coenzyme a stage. Phytochemistry 19:1459–1462
Kamsteeg J, van Brederode J, van Nigtevecht G (1976) Pleiotropic effect of a pelargonidin-hydroxylation gene in Silene dioica. Phytochemistry 15:1917–1918
Ke JY, Cole RM, Hamad EM, Hsiao YH, Cotten BM, Powell KA, Belury MA (2016) Citrus flavonoid, naringenin, increases locomotor activity and reduces diacylglycerol accumulation in skeletal muscle of obese ovariectomized mice. Mol Nutr Food Res 60:313–324
Kew Science (2020) The Royal Botanic Gardens, Great Britain. http://plantsoftheworldonline.org Accesed 27 Nov 2020
Kılınç H, Masullo M, Bottone A, Karayıldırım T, Alankuş Ö, Piacente S (2019) Chemical constituents of Silene montbretiana. Nat Prod Res 33:335–339
Kim DW, Hwang IK, Lim SS, Yoo KY, Li H, Kim YS, Kwon DY, Moon WK, Kim DW, Won MH (2009) Germinated Buckwheat extract decreases blood pressure and nitrotyrosine immunoreactivity in aortic endothelial cells in spon-taneously hypertensive rats. Phytother Res 23:993–998
Kim YB, Reed DW, Covello PS (2015) Production of triterpenoid sapogenins in hairy root cultures of Silene vulgaris. Nat Prod Commun 10:1919–1922
Kirillov V, Stikhareva T, Suleimen Y, Serafimovich M, Kabanova S, Mukanov B (2017) Chemical composition of the essential oil from carnation coniferous (Dianthus acicularis Fisch. ex Ledeb) growing wild in Northern Kazakhstan. Nat Prod Res 31:117–123
Kitanov GM (1992) Phenolic acids and flavonoids from Stellaria media (L.) Vill. (Caryophyllaceae). Pharmazie 47:470–471
Koike K, Jia Z, Nikaido T (1998) Triterpenoid saponins from Vaccaria segetalis. Phytochemistry 47:1343–1349
Koike K, Jia Z, Nikaido T (1999) New triterpenoid saponins and sapogenins from Saponaria officinalis. J Nat Prod 62:1655–1659
Kozachok S, Pecio Ł, Kolodziejczyk-Czepas J, Marchyshyn S, Nowak P, Mołdoch J, Oleszek W (2018) γ-Pyrone compounds: flavonoids and maltol glucoside derivatives from Herniaria glabra L. collected in the Ternopil region of the Ukraine. Phytochemistry 152:213–222
Kozlowska J, Potaniec B, Zarowska B, Aniol M (2017) Synthesis and biological activity of novel o-alkyl derivatives of naringenin and their oximes. Molecules 22:1–14
Krasteva IN, Popov IS, Balabanova VI, Nikolov SD, Pencheva IP (2008) Phytochemical study of Gypsophila trichotoma Wend. (Caryophyllaceae). Quim Nova 31:1125–1126
Kremer D, Košir IJ, Potočnik T, Rogulj N, Načinović K, Randić M, Srečec S, JurišićGrubešić R (2021) Phenolic compounds in two subspecies of Drypis spinosa L. (Caryophyllaceae) growing in Croatia. Acta Bot Croatica. https://doi.org/10.37427/botcro-2020-015
Krížová L, Dadáková K, Kašparovská J, Kašparovský T (2019) Isoflavones. Molecules 24:1–28
Królikowska M, Szymańska M, Wolbiś M (1983) Rhamnazin 3-rutinoside from Herniaria ciliolata Meld. spp. robusta Chaudhri. Acta Pol Pharm 40:643–648
Kubitzki K (1993) In: Kubitzki K, Rohwer JG, Bittrich V (eds) Flowering plants dicotyledons. Springer, Berlin
Kukongviriyapan U, Sompamit K, Pannangpetch P, Kukongviriyapan V, Donpunha W (2012) Preventive and therapeutic effects of quercetin on lipopolysaccharide-induced oxidative stress and vascular dysfunction in mice. Can J Physiol Pharmacol 90:1345–1353
Kulevanova S, Stefova M, Kadifkova Panovska T, Stafilov T (2003) HPLC identification and determination of myricetin, quercetin, kaempferol and total flavonoids in herbal drugs. Maced Pharm Bull 48:25–30
Kumar P, Khanna P (1994) Flavonoids from Saponaria vaccaria Linn. Indian J Plant Physiol 37:76–78
Kumar RK, Herbert C, Foster PS (2008) The “classicall” ovalbumin challenge model of asthma in mice. Curr Cancer Drug Targets 9:485–494
Kuwayama S, Nakata M, Godo T, Nakano M (2005) Analyses of anthocyanidins and anthocyanins in flower petals of Lychnis senno and its related species (Caryophyllaceae). Bull Fac Agric Niigata Univ 58:35–38
Lam KY, Ling APK, Koh RY, Wong YP, Say YH (2016) A review on medicinal properties of orientin. Adv Pharmacol Sci 2016:4104595
Larhsini M, Marston A, Hostettmann K (2003) Triterpenoid saponins from the roots of Silene cucubalus. Fitoterapia 74:237–241
Liang X, Li Y, Fan H, Huang W, Zhang H, Cui Y, Song X (2019) Chemical constituents from the roots and rhizomes of Silene tatarinowii Regel. Biochem Syst Ecol 86:103932
Lin X, Chen Y, Lv S, Tan S, Zhang S, Huang R, Huang Q (2015) Gypsophila elegans isoorientin attenuates CCl4-induced hepatic fibrosis in rats via modulation of NF-κB and TGF-β1/Smad signaling pathways. Int Immunopharmacol 28:305–312
Lin X, Wei J, Chen Y, He P, Lin J, Tan S, Huang Q (2016) Isoorientin from Gypsophila elegans induces apoptosis in liver cancer cells via mitochondrial-mediated pathway. J Ethnopharmacol 187:187–194
Litvinenko VI, Amanmuradov K, Abubakirov NK (1967) Glycosides of Vaccaria segetalis IV Isosaponarin. Chem Nat Compd 3:131–134
Liu XX, Wang L, Wang Q, Qiu B (2007) Chemical constituents from root of Psammosilene tunicoides. China J Chin Mater Med 32:921–923
Liu Z, Lindemeyer AK, Liang J, Wallner M, Shao XM, Shao Y, Olsen RW (2018) Flavonoids isolated from Tibetan medicines, binding to GABAA receptor and the anticonvulsant activity. Phytomedicine 50:1–7
Liu Y, Song FM, Ma ST, Moro A, Feng WY, Liao SJ, Liu Q (2019) Vaccarin prevents titanium particle-induced osteolysis and inhibits RANKL-induced osteoclastogenesis by blocking NF-κB and MAPK signaling pathways. J Cell Physiol 234:13832–13842
Luo JG, Cao LH, Kong LY (2012) Two new β-carboline-type alkaloids from Stellaria dichotoma var. lanceolata. Chin Chem Lett 23:1385–1388
Ma X, Wu C, Wang W, Li X (2006) Peptides form plants: a new source for antitumor drug research. Asian J Tradit Med 1:85–90
Ma L, Gu YC, Luo JG, Wang JS, Huang XF, Kong LY (2009) Triterpenoid saponins from Dianthus versicolor. J Nat Prod 72:640–644
Ma LY, Liu RH, Xu XD, Yu MQ, Zhang Q, Liu HL (2010) The pharmacokinetics of C-glycosyl flavones of Hawthorn leaf flavonoids in rat after single dose oral administration. Phytomedicine 17:640–645
Maleš Ž, Crkvenčić M, Pilepić KH, Herenda F (2013) Investigation of flavonoids, phenolic acids and amino acids of smooth rupturewort—Herniaria glabra L. Farm Glas 69:673–684
Maliński MP, Michalska AD, Tomczykowa M, Tomczyk M, Thiem B (2014) Ragged Robin (Lychnis flos-cuculi)—a plant with potential medicinal value. Rev Bras 24:722–730
Maliński MP, Kikowska M, Kruszka D, Napierała M, Florek E, Śliwińska E, Thiem B (2019) Various in vitro systems of Ragged Robin (Lychnis fos-cuculi L.): a new potential source of phytoecdysteroids? Plant Cell Tissue Organ Cult 139:39–52
Mamadalieva NZ, Zibareva LN, Lafont R, Dainan L, Saatov Z (2004) Phytoecdysteroids from the Silene genus. Chem Nat Compd 40:574–578
Mamadalieva NZ, Egamberdieva D, Lafont R, Girault JP (2008) Phytoecdysteroids and antibacterial activity of the plant Coronaria flos-cuculi. Chem Nat Compd 44:404–406
Mamadalieva NZ, El-Readi MZ, Janibekov AA, Tahrani A, Wink M (2011) Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant activity. Z Naturforsch 66C:215–224
Mamadalieva NZ, Lafont R, Wink M (2014) Diversity of secondary metabolites in the genus Silene L. (Caryophyllaceae)—structures, distribution, and biological properties. Diversity 6:415–499
Mandal P, Misra TK, Ghosal M (2009) Free-radical scavenging activity and phytochemical analysis in the leaf and stem of Drymaria diandra Blume. Int J Integr Biol 7:80–84
Martineti V, Tognarini I, Azzari C, Sala SC, Clematis F, Dolci M, Curir P (2010) Inhibition of in vitro growth and arrest in the G0/G1 phase of HCT8 line human colon cancer cells by kaempferide triglycoside from Dianthus caryophyllus. Phytother Res 24:1302–1308
Mbark AN, Charrouf Z, Guillaume D, Kol O (1999) New glycosides from Herniaria fontanesii. Stud Plant Sci 6:314–319
Melnyk MV, Vodoslavskyi VM, Obodianskyi MA (2018) Research of phenolic compounds of Ruta graveolens L. and Stellaria media (L.) Vill. Asian J Pharm Clin Res 11:152–156
Meng Y, Whiting P, Zibareva L, Bertho G, Girault JP, Lafont R, Dinan L (2001) Identification and quantitative analysis of the phytoecdysteroids in Silene species (Caryophyllaceae) by high-performance liquid chromatography: Novel ecdysteroids from S. pseudotites. J Chromatogr A 935:309–319
Meselhy MR, Aboutabl ES (1997) Hopane-type saponins from Polycarpon succulentum growing in Egypt. Phytochemistry 44:925–929
Mihaylova D, Vrancheva R, Desseva I, Ivanov I, Dincheva I, Popova M, Popova A (2018) Analysis of the GC-MS of volatile compounds and the phytochemical profile and antioxidant activities of some Bulgarian medicinal plants. Z Naturforschung 74C:45–54
Mikšátková P, Ancheeva E, Hejtmánková K, Teslov L, Lapčík O (2014) Determination of flavonoids in Stellaria by high-performance liquid chromatography-tandem mass spectrometry. Anal Lett 47:2317–2331
Morales P, Carvalho AM, Sánchez-Mata MC, Cámara M, Molina M, Ferreira ICFR (2012) Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet Resour Crop Evol 59:851–863
Mubarek MM (2019) Pharmacognostical studies on Gymnocarpos decandrus Forrssk. growing at North Western Coast in Egypt, Doctoral dissertation, Cairo University
Nakano T, Sugimoto S, Matsunami K, Otsuka H (2011) Dianthosaponins A-F, triterpene saponins, flavonoid glycoside, aromatic amide glucoside and γ-pyrone glucoside from Dianthus japonicus. Chem Pharm Bull 59:1141–1148
Nakayama M, Koshioka M, Yoshida H, Kan Y, Fukui Y, Koike A, Yamaguchi MA (2000) Cyclic malyl anthocyanins in Dianthus caryophyllus. Phytochemistry 55:937–939
Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: A review. Biores Technol 101:372–378
Niemann GJ (1984) Leaf flavonoid glycosylation and sprout morphogenesis in Silene pratensis influenced by the spectral composition of light. J Plant Physiol 115:311–318
Niemann GJ (1993) The anthranilamide phytoalexins of the Caryophyllaceae and related compounds. Phytochemistry 34:319–328
Nono RN, Nguelefack-Mbuyo EP, Nzowa LK, Ponou BK, Teponno RB, Nguelefack TB, Park HJ (2016) Antioxidant C-glycosylflavones of Drymaria cordata (Linn.) Willd. Arch Pharmacal Res 39:43–50
Obmann A, Zehl M, Purevsuren S, Narantuya S, Reznicek G, Kletter C, Glasl S (2011b) Quantification of flavonoid glycosides in an aqueous extract from the traditional Mongolian medicinal plant Dianthus versicolor Fisch. J Sep Sci 34:292–298
Obmann A, Werner I, Presser A, Zehl M, Swoboda Z, Purevsuren S, Glasl S (2011a) Flavonoid C- and O-glycosides from the Mongolian medicinal plant Dianthus versicolor Fisch. Carbohyd Res 346:1868–1875
Obmann A, Purevsuren S, Zehl M, Kletter C, Reznicek G, Narantuya S, Glasl S (2012) HPLC determination of flavonoid glycosides in Mongolian Dianthus versicolor Fisch. (Caryophyllaceae) compared with quantification by UV spectrophotometry. Phytochem Anal 23:254–259
Ogata J, Itoh Y, Ishida M, Yoshida H, Ozeki Y (2004) Cloning and heterologous expression of cDNAs encoding flavonoid glucosyltransferases from Dianthus caryophyllus. Plant Biotechnol 21:367–375
Olennikov DN (2020) Silenerepin—a new C-glycosylflavone from Silene repens. Chem Nat Compd 56:423–426
Pacifico S, Scognamiglio M, D’Abrosca B, Piccolella S, Tsafantakis N, Gallicchio M, Fiorentino A (2010) Spectroscopic characterization and antiproliferative activity on HepG2 human hepatoblastoma cells of flavonoid C-glycosides from Petrorhagia velutina. J Nat Prod 73:1973–1978
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:1–15
Pei R, Liu X, Bolling B (2020) Flavonoids and gut health. Curr Opin Biotechnol 61:153–159
Peng Y, Gan R, Li H, Yang M, Mcclements DJ (2020) Absorption, metabolism, and bioactivity of vitexin: recent advances in understanding the efficacy of an important nutraceutical. Critical Reviews in Food Science and Nutrition 1–16
Plant Database (2020) United States Department of Agriculture, United States of America. https://plants.sc.egov.usda.gov. Accesed 27 Nov 2020
Powers JM, Seco R, Faiola CL, Sakai AK, Weller SG, Campbell DR, Guenther A (2020) Floral scent composition and fine-scale timing in two moth-pollinated Hawaiian Schiedea (Caryophyllaceae). Front Plant Sci 11:1–16
Praveena R, Sadasivam K, Deepha V, Sivakumar R (2014) Antioxidant potential of orientin: a combined experimental and DFT approach. J Mol Struct 1061:114–123
Qi P, Li Z, Chen M, Sun Z, Huang C (2013) Metabolism and tissue distribution study of Vaccaria seeds (Wang-Bu-Liu-Xing) in benign prostatic hyperplasia model rat: toward an in-depth study for its bioactive components. J Pharm Biomed Anal 85:218–230
Qi P, Zhang F, Xue R, Li Z, Chen M, Sun Z, Huang C (2014) Identification of multiple constituents from seed of Vaccaria segetalis with an adsorbent-separation strategy based on liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 28:1243–1257
Radulović NS, Ristić MN, Ristić NR, Dekić VS, Dekić BR, Mladenović MZ (2018) The floral scent of Dianthus cruentus Griseb (Caryophyllaceae). Fac Univ Ser Phys Chem Technol 16:161
Richardson M (1978) Flavonols and C-glycosylflavonoids of the caryophyllales. Biochem Syst Ecol 6:283–286
Rizk AM (1986) Phytochemistry of the flora of Qatar. Scientific and Applied Research Centre, University of Qatar, Qatar
Rogowska M, Lenart M, Srecec S, Ziaja M, Parzonko A, Bazylko A (2017) Chemical composition, antioxidative and enzyme inhibition activities of chickweed herb (Stelaria media L., Vill.) ethanolic and aqueous extracts. Ind Crops Prod 97:448–454
Sachdeva AK, Kuhad A, Chopra K (2014) Naringin ameliorates memory deficits in experimental paradigm of Alzheimer’s disease by attenuating mitochondrial dysfunction. Pharmacol Biochem Behav 127:101–110
Said RB, Hamed AI, Masullo M, Al-Ayed AS, Moustafa MFM, Mahalel UA, Piacente S (2019) Flavone C-glycosides from Vaccaria pyramidata: structure elucidation by spectroscopy and theoretical calculations. Phytochem Lett 29:119–124
Sait S, Hamri-Zeghichi S, Boulekbache-Makhlouf L, Madani K, Rigou P, Brighenti V, Pellati F (2015) HPLC-UV/DAD and ESI-MSn analysis of flavonoids and antioxidant activity of an Algerian medicinal plant: Paronychia argentea Lam. J Pharm Biomed Anal 111:231–240
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, Sharifi-Rad J (2019) The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 12:1–18
Salt TA, Adler JH (1986) Dominance of Δ7-sterols in the family Caryophyllaceae. Lipids 21:754–758
Sang SM, Xia ZH, Mao SL, Lao A, Chen ZL (2000) Studies on the flavonol glycosides from the seeds of Vaccaria segetalis. China J Chin Mater Med 25:221–222
Sang S, Xia Z, Lao A, Cao L, Chen Z, Uzawa J, Fujimoto Y (2003b) Studies on the constituents of the seeds of Vaccaria segetalis. Heterocycles 59:811–821
Sang S, Lao A, Chen Z, Uzawa J, Fujimoto Y (2003) In: Ho CT (ed.) Oriental foods and herbs. Oxford University Press, Washington
Schmidt J, Bohme F, Adam G (1996) 24-Epibrassinolide from Gypsophila perfoliata. Z Naturforsch 51C:897–899
Schweingruber FH (2007) Stem anatomy of Caryophyllaceae. Flora - Morphology, Distribution, Functional Ecology of Plants 202:281–292
Seo C, Shin HS, Lee JE, Jung YW, Kim JK, Kwon JG, Hong SS (2020) Isolation and structure elucidation of siliendines A-D, new β-carboline alkaloids from Silene seoulensis. Phytochem Lett 36:58–62
Seraya L, Birke K, Khimenko SV, Boguslavskaya L (1978) Flavonoid compounds of Dianthus superbus. Khim Prir Soedin 6:802–803
Serra A, Macià A, Romero MP, Reguant J, Ortega N, Motilva MJ (2012) Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 130:383–393
Shafaghat A, Shafaghatlonbar M (2011) Antimicrobial activity and chemical constituents of the essential oils from flower, leaf and stem of Gypsophila bicolor from Iran. Nat Prod Commun 6:275–276
Sharma A, Arora D (2012) Phytochemical and pharmacological potential of genus Stellaria: A review. J Pharm Res 5:3591–3596
Shinjiro O, Junko M, Godo T, Kato Y (2009) Possibility for selective accumulation of polyphenolics in tissue cultures of Senno (Lychnis senno Siebold et Zucc.). Nat Prod Commun 4:377–380
Shukla S, Gupta S (2010) Apigenin: a promising molecule for cancer prevention. Pharm Res 27:962–978
Simeonova R, Kondeva-Burdina M, Vitcheva V, Krasteva I, Manov V, Mitcheva M (2014) Protective effects of the apigenin-O/C-diglucoside saponarin from Gypsophila trichotoma on carbone tetrachloride-induced hepatotoxicity in vitro/in vivo in rats. Phytomedicine 21:148–154
Singh M, Kaur M, Silakari O (2014) Flavones: an important scaffold for medicinal chemistry. Eur J Med Chem 84:206–239
Slavokhotova AA, Odintsova TI, Rogozhin EA, Musolyamov AK, Andreev YA, Grishin EV, Egorov TA (2011) Isolation, molecular cloning and antimicrobial activity of novel defensins from common chickweed (Stellaria media L.) seeds. Biochimie 93:450–456
Smolyakova IM, Avdeenko SN, Kalinkina GI, Yusubov MS, Zibareva LN (2010) Analysis of the chemical composition of Lychnis chalcedonica cultivated in Western Siberia. Chem Plant Mater 2010:95–102
Stich K, Eidenberger T, Wurst F, Forkmann G (1992) Enzymatic conversion of dihydroflavonols to flavan-3,4-diols using flower extracts of Dianthus caryophyllus L. (carnation). Planta 187:103–108
Sun J, Yu JH, Song JL, Jiang CS, Yuan T, Zhang H (2019) Two new quinolone alkaloids from Dianthus superbus var. superbus. Tetrahedron Lett 60:161–163
Taskin T, Bitis L (2013) Antioxidant activity of Silene alba subsp. divaricata and Stellaria media subsp. media from Caryophyllaceae. Spatula DD 3:1–5
Thiem B, Kikowska M, Maliński MP, Kruszka D, Napierała M, Florek E (2016) Ecdysteroids: production in plant in vitro cultures. Phytochem Rev 16:603–622
Thilakarathna SH, Rupasinghe HPV (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5:3367–3387
Tlili H, Hanen N, Arfa AB, Neffati M, Boubakri A, Buonocore D, Doria E (2019) Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 14:1–18
Tomczyk M (2008) Preliminary phytochemical investigation of Lychnis flos-cuculi herbs. J Nat Med 62:473–475
Tong Y, Luo JG, Wang R, Wang XB, Kong LY (2012) New cyclic peptides with osteoblastic proliferative activity from Dianthus superbus. Bioorg Med Chem Lett 22:1908–1911
Tong H, Sun BG, ChangTao W, Sun XT, Xue Z (2014) Study on surfactant-assisted extraction process and preliminary structural analysis of total flavonoids from Arenaria kansuensis Maxim. Food Res Dev 35:14–18
Tu Y, Zhu S, Wang J, Burstein E, Jia D (2019) Natural compounds in the chemoprevention of alcoholic liver disease. Phytother Res 33:2192–2212
Ullah F, Ayaz A, Saqib S, Zaman W, Butt MA, Ullah A (2019) Silene conoidea L.: A review on its systematic, ethnobotany and phytochemical profile. Plant Sci Today 6:373–382
Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan KK, Unnikrishnan MK (2000) Radiation protection by the Ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res 154:455–460
Van Wyk BE, Wink M (2017) Medicinal plants of the World. Briza Publications, Pretoria
Van Brederode J, van Genderen HH, Berendsen W (1982) Morphological effects of the flavone isovitexin in a non-glycosylating genotype of Silene pratensis (Caryophyllaceae). Experientia 38:929–931
Van Dooren I, Foubert K, Bijttebier S, Theunis M, Velichkova S, Claeys M, Apers S (2016) Saponins and flavonoids from an infusion of Herniaria hirsuta. Planta Med 82:1576–1583
Vardavas CI, Majchrzak D, Wagner KH, Elmadfa I, Kafatos A (2006) The antioxidant and phylloquinone content of wildly grown greens in Crete. Food Chem 99:813–821
Vincken JP, Heng L, de Groot A, Gruppen H (2007) Saponins, classification and occurrence in the plant kingdom. Phytochemistry 68:275–297
Vinholes J, Grosso C, Andrade PB, Gil-Izquierdo A, Valentão P, Pinho PGD, Ferreres F (2011) In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem 129:454–462
Viskupičová J, Ondrejovič M, Šturdík E (2008) Bioavailability and metabolism of flavonoids. J Food Nutr Res 47:151–162
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 38:15–25
Vitcheva V, Simeonova R, Krasteva I, Yotova M, Nikolov S, Mitcheva M (2011) Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats. Redox Rep 16:56–61
Volodin VV, Volodina SO (2015) Floristic and molecular phylogenetic analysis of the distribution of phytoecdysteroids among plants of North-East Russia (Asteraceae and Caryophyllaceae). Biol Med 7:1
Wang X, Dong H, Liu Y, Yang B, Wang X, Huang L (2011) Application of high-speed counter-current chromatography for preparative separation of cyclic peptides from Vaccaria segetalis. J Chromatogr B 879:811–814
Wang G, Luo JG, Yang MH, Wang XB, Kong LY (2013) Six new cyclic peptides from the roots of Gypsophila oldhamiana. Chem Pharm Bull 61:489–495
Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, Gao Y (2016) The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol 56:21–38
Wink M (2011) Biochemistry of Plant Secondary Metabolism, 2nd edn. Wiley-Blackwell, Chichester
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines 2:251–286
Wolf SJ, Denford KE, Packer JG (1979) A study of the flavonoids of the Minuartia rossii complex. Can J Bot 57:2374–2377
Wu FE, Koike K, Nikaido T, Sakamoto Y, Ohmoto T, Ikeda K (1989) New β-Carboline alkaloids from a Chinese medicinal plant, Arenaria kansuensis. Structures of arenarines A, B, C, D. Chem Pharm Bull 37:1808–1809
Wu FE, Koike K, Nikaido T, Ishii K, Ohmoto T, Ikeda K (1990) Terpenoids and flavonoids from Arenaria kansuensis. Chem Pharm Bull 38:2281–2282
Yasukawa K, Yamanouchi S, Takido M (1981) Studies on the constituents in the water extracts of crude drugs. III. On the roots of Stellaria dichotoma L. var. lanceolata BGE. Yakugaku Zasshi 101:64–66
Yayli N, Seymen H, Baltaci C (2001) Flavone C-glycosides from Scleranthus uncinatus. Phytochemistry 58:607–610
Yayli N, Baltaci C, Genç H, Terzioǧlu S (2002) Phenolic and flavone C-glycosides from Scleranthus uncinatus. Pharm Biol 40:369–373
Yoshida H, Itoh Y, Ozeki Y, Iwashina T, Yamaguchi MA (2004) Variation in chalcononaringenin 2′-O-glucoside content in the petals of carnations (Dianthus caryophyllus) bearing yellow flowers. Sci Hortic 99:175–186
Yücel TB, Yayli N (2018) GC/MS analysis and antimicrobial activity of the volatile compounds from Dianthus carmelitarum Reut. ex Boiss and Dianthus calocephalus Boiss. grown in Turkey. J Agric Fac Ege Univ 55:89–94
Zanotti SD, de Abreu Ribeiro GK, Zeppone LC, Borges CT (2013) Orange juice and hesperidin promote differential innate immune response in macrophages ex vivo. Int J Vitam Nutr Res 83:162–167
Zaychenko SG, Zernov AS (2017) Structural features of the seed coat in Caucasian representatives of Minuartia (Caryophyllaceae ). Wulfenia J 24:205–220
Zdraveva P, Gevrenova R, Dimitrova B (2004) Phenolic compounds of Scleranthus annuus L. (Caryophyllaceae). 3rd Conference on Medicinal and Aromatic Plants of Southeast European Countries. Nitra, Slovakia Republic 2004:57
Zdraveva P, Pencheva I, Popova P, Ionkova I, Krasteva I (2015) Production of saponarin in in vitro cultures of Gypsophila species. J Chem Pharm Res 7:829–832
Zemtsova GN, Glyzin VY, Dzhumyrko SF (1976) Flavones and their C-glycosides from Silene saxatilis. Chem Nat Compd 11:538
Zhang H (2012) Profiling analysis of the seeds of Vaccaria segetalis (Necr.) Gracke by HPLC-ESI-MS. Adv Mater Res 396–398:96–98
Zhang FM, Tai ZG, Cai L, Yang YB, Li F, Ding ZT (2011) Flavonoids from Gypsophila elegans and their antioxidant activities. J Yunnan Univ (Nat Sci Ed) 33:93–95
Zhang H, Wang K, Wu J, Chen Y, He P (2011) A new flavonoid glycoside from Vaccaria hispanica. Nat Prod Commun 6:1599–1602
Zhang Y, Wang G, Lv H, Luo J, Kong L (2015) Two new β-carboline alkaloids from the roots of Gypsophila oldhamiana. Nat Prod Res 29:1207–1211
Zheleva-Dimitrova D, Zengin G, Balabanova V, Voynikov Y, Lozanov V, Lazarova I, Gevrenova R (2018) Chemical characterization with in vitro biological activities of Gypsophila species. J Pharm Biomed Anal 155:56–69
Zhou X, Wang L, Tian Y, Gong X, Zhao C, Yang S (2013) Chemical constituents from roots of Psammosilene tunicoides. Zhongguo Zhong Yao Za Zhi 38:3507–3509
Zhou G, Tang L, Wang T, Zhou X, Kou Z, Wu J, Wang Z (2016) Phytochemistry and pharmacological activities of Vaccaria hispanica (Miller) Rauschert: a review. Phytochem Rev 15:813–827
Zhou G, Wu H, Wang T, Guo R, Xu J, Zhang Q, Wang Z (2017) C-glycosylflavone with rotational isomers from Vaccaria hispanica (Miller) Rauschert seeds. Phytochem Lett 19:241–247
Zhu G, Liu X, Li H, Yan Y, Hong X, Lin Z (2018) Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int J Immunopathol Pharmacol 32:1–12
Zhuang L (1983) C-Glycosylflavones from Qi Gu Cao (Sagina japonica). Zhongcaoyao 14:295–297
Zitouni M (2017) Profil polyphénolique et activité antioxydante de deux plantes médicinales Pistacia lentiscus. L et Gymnocarpos decander Forsk. Universite Abou Bekr Belkaid, Tlemcen
Zoll A, Nouvel G (1974) Comparative study of C-glycosyl flavones of two Caryophyllaceae. Spergularia rubra and Stellaria holostea. Phytochemistry 8:134–140
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology, K.J., M.T.; Formal Analysis, K.J.; Investigation, K.J.; Writing – Original Draft Preparation, K.J.; Writing – Review and Editing, M.T., M.W.; Supervision, M.T.; Project Administration, M.T.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jakimiuk, K., Wink, M. & Tomczyk, M. Flavonoids of the Caryophyllaceae. Phytochem Rev 21, 179–218 (2022). https://doi.org/10.1007/s11101-021-09755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-021-09755-3