Abstract
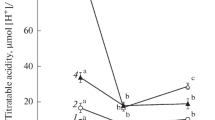
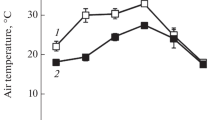
The effects of the diurnal variations in ambient temperature on some C3 and C4 enzymes in the Salsola dendroides and Suaeda altissima species of Chenopodiaceae family were studied during the intensive vegetation period. Activities of phosphoenolpyruvate carboxylase (PEPC) and cytosolic aspartate aminotransferase (AsAT) were shown to decrease in both species in the afternoon and evening. The activity of the mitochondrial AsAT decreased in S. altissima, remained relatively constant in S. dendroides during the day. The activity of alanine aminotransferase was high in the S. dendroides species in the morning and evening and decreased in the S. altissima species by the evening. Glucose-6-phosphate activated PEPC in both species throughout the day. The study of the redox status-regulated C3 enzymes showed temperature-related increases in NADP-glyceraldehyde 3-phosphate dehydrogenase activity in both plants, in fructose-2,6-bisphosphatase activity in the S. altissima species, and in NADP-MDH activity in the S. dendroides species in the afternoon.
Similar content being viewed by others
Abbreviations
- AlAT:
-
alanine aminotransferase
- AsAT:
-
aspartate aminotransferase
- BS:
-
bundle sheath
- Chl:
-
chlorophyll
- EDTA:
-
ethylenediaminetetraacetic acid
- FBPase:
-
fructose-2,6-bisphosphatase
- Fv/Fm :
-
maximum quantum efficiency of PSII
- Glu-6-P:
-
glucose-6-phosphate
- M:
-
mesophyll
- MDH:
-
malate dehydrogenase
- ME:
-
malic enzyme
- 2-ME:
-
2-β-mercaptoetanol
- NADPGAPDH:
-
NADP-glyceraldehyde phosphate dehydrogenase
- PEP(C):
-
phosphoenolpyruvate (carboxylase)
- Tris:
-
tris(hydroxymethyl) aminomethane
References
Akhani H., Trimborn P., Ziegler H.: Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with their ecological, phytogeographical and taxonomical importance.‒Plant Syst. Evol. 206: 187–221, 1997.
Alfonso S.U., Brüggemann W.: Photosynthetic responses of a C3 and three C4 species of the genus Panicum (s.l.) with different metabolic subtypes to drought stress.‒Photosynth. Res. 112: 175–191, 2012.
Avasthi K., Izui K., Raghavendra A.S.: Interplay of light and temperature during the planta modulation of C4 phosphoenolpyruvate carboxylase from the leaves of Amaranthus hypochondriacus L.: diurnal and seasonal effects manifested at molecular levels.‒J. Exp. Bot. 62: 1017–1026, 2011.
Avasthi U.K., Raghavendra A.S.: Mutual stimulation of temperature and light effects on C4 phosphoenolpyruvate carboxylase in leaf discs and leaves of Amaranthus hypochondriacus.‒J. Plant Physiol. 165: 1023–1032, 2008.
Bailey K.J., Gray J.E., Walker R.P., Leegood R.C.: Coordinate regulation of phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase by light and CO2 during C4 photosynthesis.‒Plant Physiol. 144: 479–486, 2007.
Bradford M.: Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.‒Anal. Biochem. 72: 248–254, 1976.
Brestic M., Živčák M., Kunderliková K., Allakhverdiev S.I.: High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines.–Photosynth. Res. 130: 251–266, 2016.
Chinthapalli B., Murmu J., Raghavendra A.S.: Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants.‒J. Exp. Bot. 54: 707–714, 2003.
Chinthapalli B., Chitra D.S.V., Radhavendra A.S.: Temperature modulation of the activity and malate inhibition of the phosphoenolpyruvate carboxylase from leaves of Alternanthera pengens, compared to that of Lycoperisicon esculentum.‒Am. J. Biosci. 2: 238–243, 2014.
Chollet R., Vidal J., O’Leary M.H.: Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants.‒Annu. Rev. Plant Phys. 47: 273–298, 1996.
Du Y.-Ch., Nose A., Kondo A., Wasano K.: Diurnal changes in photosynthesis in sugarcane leaves. I. Carbon dioxide exchange rate, photosynthesis enzyme activities and metabolite levels relating to the C4 pathway and the Calvin cycle.‒Plant Prod. Sci. 3: 3–8, 2000.
Dwyer S.A., Ghannoum O., Nicotra A., von Caemmerer S.: High temperature acclimation of C4 photosynthesis in linked to changes in photosynthetic biochemistry.–Plant Cell Environ. 30: 53–66, 2007.
Edwards G.E., Franceschi V.R., Voznesenkaya E.V.: Single cell C4 photosynthesis versus the dual-cell (Kranz) paradigm.‒Annu. Rev. Plant Biol. 55: 173–196, 2004.
Giglioli-Guivarc’h N., Pierre J.-N., Brown S. et al.: The lightdependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis.‒Plant Cell 8: 573–586, 1996.
Gowik U., Westhoff P.: The path from C3 and C4 photosynthesis.‒Plant Physiol. 155: 56–63, 2011.
Hatch M.D.: C4 photosynthesis in a unique blend of modified biochemistry, anatomy and ultrastructure.‒BBA-Rev. Bioenergetics 895: 81–106, 1987.
Hibberd J.M., Covshoff S.: The regulation of gene expression required for C4 photosynthesis.‒Annu. Rev. Plant Biol. 61: 181–207, 2010.
Holaday A.S., Martindale W., Alred R. et al.: Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature.‒Plant Physiol. 98: 1105–1114, 1992.
Leegood R.C.: C4 photosynthesis: principles of CO2 concentration and prospects for its introduction into C3 plants.–J. Exp. Bot. 53: 581–590, 2002.
Long S.P.: Environmental responses.‒In: Sage R.F., Monson R.K. (ed.): C4 Plant Biology. Pp. 215–249. Academic Press, San Diego 1999.
Movsumova F.G., Babayev H.G., Zeynalova M.H., Feyziyev Y.M.: [Taxonomic composition of Chenopodiaceae Vent. family in Absheron flora and its ecological analysis.]‒Proc. Azerbaijan Natl. Acad. Sci. (Biol. Med. Sci.) 69: 27–35, 2014. [In Russian]
O’Leary B., Park J., Plaxton W.C.: The remarkable diversity ofplant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs.‒Biochem. J. 436: 15–34, 2011.
Pyankov V., Ziegler H., Kuz’min A., Edwards G.E.: Origin and evolution of C4 photosynthesis in the tribe Salsoleae (Chenopodiaceae) based on anatomical and biochemical types in leaves and cotyledons.‒Plant Syst. Evol. 230: 43–74, 2001.
Pyankov V.I., Voznesenskaya E.V., Kuz’min A.N. et al.: Occurrence of C3 and C4 photosynthesis in cotyledons and leaves of Salsola species (Chenopodiaceae).‒Photosynth. Res. 63: 69–84, 2000.
Rosnow J.J., Edwards G.E., Roalson E.H.: Positive selection of Kranz and non-Kranz C4 phosphoenolpyruvate carboxylase amino acids in Suaedoideae (Chenopodiaceae).–J. Exp. Bot. 65: 3595–3607, 2014.
Sage R.F., Christin P.A., Edwards E.J.: The C4 plant lineages of planet Earth.‒J. Exp. Bot. 62: 3155–3169, 2011.
Sage R.F., Kocacinar F., Kubien D.S.: C4 photosynthesis and temperature.‒In: Raghavendra A.S., Sage R.F. (ed.): C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Pp. 161–195. Springer Sci+Business Media BV, Dordrecht 2011.
Schüssler Ch., Freitag H., Koteyeva N. et al.: Molecular phylogeny and forms of photosynthesis in tribe Salsoleae (Chenopodiaceae).‒J. Exp. Bot. 68: 207–223, 2017.
Taniguchi M., Kobe M., Kato M., Sugiyama T.: Aspartate aminotransferase isozymes in Panicum miliaceum L., an NAD-Malic enzyme-type C4 plant: Comparison of enzymatic-properties, primary structures, and expression patterns.‒Arch. Biochem. Biophys. 318: 295–306, 1995.
Taniguchi M., Sugiyama T.: Aspartate aminotransferase from Eleusine coracana, a C4 plant: Purification, characterization, and preparation of antibody.‒Arch. Biochem. Biophys. 282: 427–432, 1990.
Yamori W., Hikosaka K., Way D.A.: Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation.‒Photosynth. Res. 119: 101–117, 2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This work was supported by the Science Development Foundation under the President of the Republic of Azerbaijan–Grant No. EIF-2012-2(6)-39/19/3.
Rights and permissions
About this article
Cite this article
Orujova, T.Y., Bayramov, S.M., Gurbanova, U.A. et al. Diurnal temperature-related variations in photosynthetic enzyme activities of two C4 species of Chenopodiaceae grown in natural environment. Photosynthetica 56, 1107–1112 (2018). https://doi.org/10.1007/s11099-018-0804-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-018-0804-x