Abstract
Purpose
Olmesartan medoxomil (olmesartan-MX), an ester-type prodrug of the angiotensin II receptor blocker (ARB) olmesartan, is predominantly anionic at intestinal pH. Human organic anion transporting polypeptide 2B1 (OATP2B1) is expressed in the small intestine and is involved in the absorption of various acidic drugs. This study was designed to test the hypothesis that OATP2B1-mediated uptake contributes to the enhanced intestinal absorption of olmesartan-MX, even though olmesartan itself is not a substrate of OATP2B1.
Methods
Tetracycline-inducible human OATP2B1- and rat Oatp2b1-overexpressing HEK 293 cell lines (hOATP2B1/T-REx-293 and rOatp2b1/T-REx-293, respectively) were established to characterize OATP2B1-mediated uptake. Rat jejunal permeability was measured using Ussing chambers. ARBs were quantified by liquid chromatography-tandem mass spectrometry.
Results
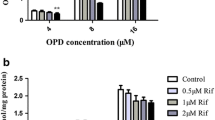
Significant olmesartan-MX uptake was observed in hOATP2B1/T-REx-293 and rOatp2b1/T-REx-293 cells, whereas olmesartan uptake was undetectable or much lower than olmesartan-MX uptake, respectively. Furthermore, olmesartan-MX exhibited several-fold higher uptake in Caco-2 cells and greater permeability in rat jejunum compared to olmesartan. Olmesartan-MX uptake in hOATP2B1/T-REx-293 cells and in Caco-2 cells was significantly decreased by OATP2B1 substrates/inhibitors such as 1 mM estrone-3-sulfate, 100 µM rifamycin SV, and 100 µM fluvastatin. Rat Oatp2b1-mediated uptake and rat jejunal permeability of olmesartan-MX were significantly decreased by 50 µM naringin, an OATP2B1 inhibitor. Oral administration of olmesartan-MX with 50 µM naringin to rats significantly reduced the area under the plasma concentration–time curve of olmesartan to 76.9%.
Conclusion
Olmesartan-MX is a substrate for OATP2B1, and the naringin-sensitive transport system contributes to the improved intestinal absorption of olmesartan-MX compared with its parent drug, olmesartan.




Similar content being viewed by others
Abbreviations
- candesartan-CX:
-
Candesartan cilexetil
- OATP/Oatp:
-
Organic anion transporting polypeptide
- olmesartan-MX:
-
Olmesartan medoxomil
References
Martinez MN, Amidon GL. A Mechanistic Approach to Understanding the Factors Affecting Drug Absorption: A Review of Fundamentals. J Clin Pharmacol. 2002;42:620–43.
Matsson P, Doak BC, Over B, Kihlberg J. Cell permeability beyond the rule of 5. Adv Drug Deliv Rev. 2016;101:42–61.
Doak BC, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem Biol. 2014;21:1115–42.
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70.
Waring MJ. Lipophilicity in drug discovery. Expert Opin Drug Discov. 2010;5:235–48.
Cundy KC, Branch R, Chernov-Rogan T, Dias T, Estrada T, Hold K, et al. XP13512 [(±)-1-([(α-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311:315–23.
Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of Valganciclovir, a Ganciclovir Prodrug, via Peptide Transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89:781–9.
Epling D, Hu Y, Smith DE. Evaluating the intestinal and oral absorption of the prodrug valacyclovir in wildtype and huPepT1 transgenic mice. Biochem Pharmacol. 2018;155:1–7.
Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, et al. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm. 2010;7:1747–56.
Kobayashi D, Nozawa T, Imai K, Nezu JI, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–8.
Keiser M, Kaltheuner L, Wildberg C, Müller J, Grube M, Partecke LI, et al. The Organic Anion-Transporting Peptide 2B1 Is Localized in the Basolateral Membrane of the Human Jejunum and Caco-2 Monolayers. J Pharm Sci. 2017;106:2657–63.
Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20.
Shirasaka Y, Shichiri M, Mori T, Nakanishi T, Tamai I. Major active components in grapefruit, orange, and apple juices responsible for OATP2B1-mediated drug interactions. J Pharm Sci. 2013;102:280–8.
Johnson M, Patel D, Matheny C, Ho M, Chen L, Ellens H. Inhibition of intestinal OATP2B1 by the calcium receptor antagonist ronacaleret results in a significant drug-drug interaction by causing a 2-fold decrease in exposure of rosuvastatin. Drug Metab Dispos. 2017;45:27–34.
Kondo A, Narumi K, Okuhara K, Takahashi Y, Furugen A, Kobayashi M, et al. Black tea extract and theaflavin derivatives affect the pharmacokinetics of rosuvastatin by modulating organic anion transporting polypeptide (OATP) 2B1 activity. Biopharm Drug Dispos. 2019;40:302–6.
Medwid S, Li MMJ, Knauer MJ, Lin K, Mansell SE, Schmerk CL, et al. Fexofenadine and rosuvastatin pharmacokinetics in mice with targeted disruption of organic anion transporting polypeptide 2b1. Drug Metab Dispos. 2019;47:832–42.
Chen M, Hu S, Li Y, Gibson AA, Fu Q, Baker SD, et al. Role of OATP2B1 in drug absorption and drug-drug interactions. Drug Metab Dispos. 2020;48:419–25.
Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2011;165:1260–87.
Kinzi J, Grube M, Meyer zu Schwabedissen HE. OATP2B1 – The underrated member of the organic anion transporting polypeptide family of drug transporters? Biochem Pharmacol. 2021;188:114534. https://doi.org/10.1016/j.bcp.2021.114534.
Lima LM, da Silva BNM, Barbosa G, Barreiro EJ. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020;208: 112829.
Shah K, Gupta JK, Chauhan NS, Upmanyu N, Shrivastava SK, Mishra P. Prodrugs of NSAIDs: A Review. Open Med Chem J. 2017;11:146–95.
Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000;57:S3-7.
Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. 2013;65:809–48.
Ettmayer P, Amidon GL, Clement B, Testa B. Lessons Learned from Marketed and Investigational Prodrugs. J Med Chem. 2004;47:2393–404.
Najjar A, Karaman R. The prodrug approach in the era of drug design. Expert Opin Drug Deliv. 2019;16:1–5.
Stella VJ. Prodrugs: Some thoughts and current issues. J Pharm Sci. 2010;99:4755–65.
US Food and Drug Administration. Advancing Health Through Innovation: New Drug Therapy Approvals 2019 [cited 2023 Oct 20]. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019.
US Food and Drug Administration. Advancing Health Through Innovation: New Drug Therapy Approvals 2020. [cited 2023 Oct 20]. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020.
US Food and Drug Administration. Advancing Health Through Innovation: New Drug Therapy Approvals 2021 [cited 2023 Oct 20]. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021.
US Food and Drug Administration. Advancing Health Through Innovation: New Drug Therapy Approcals 2022 [cited 2023 Oct 20]. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022.
Bednarczyk D, Sanghvi MV. Organic anion transporting polypeptide 2B1 (OATP2B1), an expanded substrate profile, does it align with OATP2B1’s hypothesized function? Xenobiotica. 2020;50:1128–37.
Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35:2166–76.
Yanagisawa H, Koike H. Discovery of new AT1 receptor blocker Olmesartan Medoxomil. Medchem News. 2006;16:23–7.
Noguchi S, Nishimura T, Fujibayashi A, Maruyama T, Tomi M, Nakashima E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J Pharm Sci. 2015;104:3128–35.
Tamai I, Nezu JI, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–60.
Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–38.
Noguchi S, Nishimura T, Mukaida S, Benet LZ, Nakashima E, Tomi M. Cellular Uptake of Levocetirizine by Organic Anion Transporter 4. J Pharm Sci. 2017;106:2895–8.
Nishimura T, Kato Y, Amano N, Ono M, Kubo Y, Kimura Y, et al. Species difference in intestinal absorption mechanism of etoposide and digoxin between cynomolgus monkey and rat. Pharm Res. 2008;25:2467–76.
Noguchi S, Okochi M, Atsuta H, Kimura R, Fukumoto A, Takahashi K, et al. Substrate recognition of renally eliminated angiotensin II receptor blockers by organic anion transporter 4. Drug Metab Pharmacokinet . 2021;36. https://doi.org/10.1016/j.dmpk.2020.10.002.
Hoshino Y, Fujita D, Nakanishi T, Tamai I. Molecular localization and characterization of multiple binding sites of organic anion transporting polypeptide 2B1 (OATP2B1) as the mechanism for substrate and modulator dependent drug-drug interaction. Medchemcomm. 2016;7:1775–82.
Lan T, Rao A, Haywood J, Davis CB, Han C, Garver E, et al. Interaction of macrolide antibiotics with intestinally expressed human and rat organic anion-transporting polypeptides. Drug Metab Dispos. 2009;37:2375–82.
Shirasaka Y, Li Y, Shibue Y, Kuraoka E, Spahn-Langguth H, Kato Y, et al. Concentration-dependent effect of naringin on intestinal absorption of beta(1)-adrenoceptor antagonist talinolol mediated by p-glycoprotein and organic anion transporting polypeptide (Oatp). Pharm Res. 2009;26:560–7.
Hathout RM, Elshafeey AH. Development and characterization of colloidal soft nano-carriers for transdermal delivery and bioavailability enhancement of an angiotensin II receptor blocker. Eur J Pharm Biopharm. 2012;82:230–40.
Schwocho LR, Masonson HN. Pharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjects. J Clin Pharmacol. 2001;41:515–27.
Shirasaka Y, Mori T, Murata Y, Nakanishi T, Tamai I. Substrate- and dose-dependent drug interactions with grapefruit juice caused by multiple binding sites on OATP2B1. Pharm Res. 2014;31:2035–43.
Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, et al. PH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011;8:1303–13.
Unger MS, Mudunuru J, Schwab M, Hopf C, Drewes G, Nies AT, et al. Clinically Relevant OATP2B1 Inhibitors in Marketed Drug Space. Mol Pharm. 2020;17:488–98.
Shirasaka Y, Mori T, Shichiri M, Nakanishi T, Tamai I. Functional pleiotropy of organic anion transporting polypeptide OATP2B1 due to multiple binding sites. Drug Metab Pharmacokinet. 2012;27:360–4.
Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther. 2010;332:181–9.
Tourniaire F, Hassan M, André M, Ghiringhelli O, Alquier C, Amiot MJ. Molecular mechanisms of the naringin low uptake by intestinal Caco-2 cells. Mol Nutr Food Res. 2005;49:957–62.
Lindahl A, Sjöberg A, Bredberg U, Toreson H, Ungell AL, Lennernäs H. Regional intestinal absorption and biliary excretion of fluvastatin in the rat: possible involvement of mrp2. Mol Pharm. 2004;1:347–56.
Biganzoli E, Cavenaghi LA, Rossi R, Brunati MC, Nolli ML. Use of a Caco-2 cell culture model for the characterization of intestinal absorption of antibiotics. Il Farmaco. 1999;54:594–9.
Fardel O, Lecureur V, Loyer P, Guillouzo A. Rifampicin enhances anti-cancer drug accumulation and activity in multidrug-resistant cells. Biochem Pharmacol. 1995;49:1255–60.
Walters HC, Craddock AL, Fusegawa H, Willingham MC, Dawson PA. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am J Physiol Gastrointest Liver Physiol. 2000;279:42–6.
Arakawa H, Shirasaka Y, Haga M, Nakanishi T, Tamai I. Active intestinal absorption of fluoroquinolone antibacterial agent ciprofloxacin by organic anion transporting polypeptide, Oatp1a5. Biopharm Drug Dispos. 2012;33:332–41.
Hanafy S, El-Kadi AOS, Jamali F. Effect of Inflammation on Molecular Targets and Drug Transporters. J Pharm Pharm Sci. 2012;15:361–75.
MacLean C, Moenning U, Reichel A, Fricker G. Regional absorption of fexofenadine in rat intestine. Eur J Pharm Sci. 2010;41:670–4.
Kang MJ, Kim HS, Jeon HS, Park JH, Lee BS, Ahn BK, et al. In situ intestinal permeability and in vivo absorption characteristics of olmesartan medoxomil in self-microemulsifying drug delivery system. Drug Dev Ind Pharm. 2012;38:587–96.
Shim C-K, Cheon E-P, Kang KW, Seo K-S, Han H-K. Inhibition effect of flavonoids on monocarboxylate transporter 1 (MCT1) in Caco-2 cells. J Pharm Pharmacol. 2010;59:1515–9.
Wenzel U, Kuntz S, Diestel S, Daniel H. PEPT1-mediated cefixime uptake into human intestinal epithelial cells is increased by Ca2+ channel blockers. Antimicrob Agents Chemother. 2002;46:1375–80.
Li X, Sun J, Guo Z, Zhong D, Chen X. Carboxylesterase 2 and intestine transporters contribute to the low bioavailability of allisartan, a prodrug of exp3174 for hypertension treatment in humans. Drug Metab Dispos. 2019;47:843–53.
Funding
This work was supported in part by JSPS KAKENHI grant numbers 18K06900, 21H02651, and 22K06750, as well as by JST SPRING, Grant Number JPMJSP2123 and AMED CREST, Grant Number JP20gm1310009. It was also funded in part by the Keio Gijuku Academic Development Fund, the Fukuzawa Memorial Fund, the Hoansha Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fukazawa, N., Nishimura, T., Orii, K. et al. Conversion of Olmesartan to Olmesartan Medoxomil, A Prodrug that Improves Intestinal Absorption, Confers Substrate Recognition by OATP2B1. Pharm Res (2024). https://doi.org/10.1007/s11095-024-03687-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-024-03687-1