Abstract
Purpose
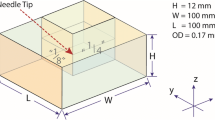
Many monoclonal antibodies (mAbs) are administered via subcutaneous (SC) injection. Local transport and absorption kinetics and mechanisms, however, remain poorly understood. A multiphysics computational model was developed to simulate the injection and absorption processes of a protein solution in the SC tissue.
Methods
Quantitative relationships among tissue properties and transport behaviors of an injected solution were described by respective physical laws. SC tissue was treated as a 3-dimensional homogenous, poroelastic medium, in which vasculatures and lymphatic vessels were implicitly treated. Tissue deformation was considered, and interstitial fluid flow was modeled by Darcy’s law. Transport of the drug mass was described based on diffusion and advection, which was integrated with tissue mechanics and interstitial fluid dynamics.
Results
Injection and absorption of albumin and IgG solutions were simulated. Upon injection, a sharp rise in tissue pressure, porosity, and fluid velocity could be observed at the injection tip. Largest tissue deformation appeared at the model surface. Transport of drug mass out of the injection zone was minimal. Absorption by local lymphatics was found to last several weeks.
Conclusions
A bottom-up method was developed to simulate drug transport and absorption of protein solutions in skin tissue base on physical principles. The results appear to match experimental observations.












Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- FEM:
-
Finite element method
- HSA:
-
Human serum albumin
- IV:
-
Intravenous
- mAbs:
-
Monoclonal antibodies
- OD:
-
Outer diameter
- PK:
-
Pharmacokinetic
- SC:
-
Subcutaneous
References
Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev. 2017;122:2–19.
Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12(1):1703531.
Tachibana T, Kuwabara T. Antibody-based therapeutics. Drug Metabolism and Pharmacokinetics. 2019;34(1):1–2.
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14.
The top selling prescription drugs by revenue. In. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/features/top-selling-prescription-drugs/; 2019.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70.
Viola M, Sequeira J, Seiça R, Veiga F, Serra J, Santos AC, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release Off J Control Release Soc. 2018;286:301–14.
Awwad S, Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018;10(3).
McDowell A, Medlicott NJ. Anatomy and physiology of the injection site: implications for extended release parenteral systems. In: Wright JC, Burgess DJ, editors. Long acting injections and implants: Springer US; 2012. p. 57–71.
Kansara V, Mitra A, Wu Y. Subcutaneous delivery of small molecule formulations: an insight into biopharmaceutics & formulation strategies. Drug Delivery Technology. 2009;9:38–43.
Turner MR, Balu-Iyer SV. Challenges and opportunities for the subcutaneous delivery of therapeutic proteins. J Pharm Sci. 2018;107(5):1247–60.
Papadmitriou K, Trinh XB, Altintas S, Van Dam PA, Huizing MT, Tjalma WAA. The socio-economical impact of intravenous (IV) versus subcutaneous (SC) administration of trastuzumab: future prospectives. Facts Views Vis Obgyn. 2015;7(3):176–80.
Ashai NI, Paganini EP, Wilson JM. Intravenous versus subcutaneous dosing of epoetin: a review of the literature. Am J Kidney Dis. 1993;22(2 Suppl 1):23–31.
Patel TV, Robinson K, Singh AK. Is it time to reconsider subcutaneous administration of epoetin? Nephrol News Issues. 2007;21(11):57.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–40.
Baert L, van 't Klooster G, Dries W, Francois M, Wouters A, Basstanie E, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. European Journal of Pharmaceutics and Biopharmaceutics : Official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009;72(3):502–8.
Sconfienza LM, Perrone N, Lacelli F, Lentino C, Serafini G. Ultrasound-guided injection of botulinum toxin a in the treatment of iliopsoas spasticity. J Ultrasound. 2008;11(3):113–7.
Tanioka T, Takase K, Yashuara Y, Zhao Y, Noda C, Hisashige S, Locsin R. Efficacy and safety in intramuscular injection techniques using ultrasonographic data. In. Health: Scientific Research Publishing; 2018. p 334–350.
Kinnunen HM, Mrsny RJ. Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of the subcutaneous injection site. J Control Release Off J Control Release Soc. 2014;182:22–32.
Porter CJH, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310.
Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos Biol Fate Chem. 2014;42(11):1881–9.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40.
Sequeira JAD, Santos AC, Serra J, Estevens C, Seiça R, Veiga F, et al. Subcutaneous delivery of biotherapeutics: challenges at the injection site. Expert Opin Drug Deliv. 2019;16(2):143–51.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989;37(1):77–104.
Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res. 1990;40(2):246–63.
Stapleton S, Milosevic M, Allen C, Zheng J, Dunne M, Yeung I, et al. A mathematical model of the enhanced permeability and retention effect for liposome transport in solid tumors. PLoS One. 2013;8(12):e81157.
Zhan W, Arifin DY, Lee TK, Wang CH. Mathematical modelling of convection enhanced delivery of carmustine and paclitaxel for brain tumour therapy. Pharm Res. 2017;34(4):860–73.
Penta R, Ambrosi D, Quarteroni A. Multiscale homogenization for fluid and drug transport in vascularized malignant tissues. Mathematical Models and Methods in Applied Sciences. 2015;25(01):79–108.
Sefidgar M, Soltani M, Raahemifar K, Sadeghi M, Bazmara H, Bazargan M, et al. Numerical modeling of drug delivery in a dynamic solid tumor microvasculature. Microvasc Res. 2015;99:43–56.
Zhan W, Xu XY. A mathematical model for thermosensitive liposomal delivery of doxorubicin to solid tumour. J Drug Deliv. 2013;2013:172529.
Zhan W, Alamer M, Xu XY. Computational modelling of drug delivery to solid tumour: understanding the interplay between chemotherapeutics and biological system for optimised delivery systems. Adv Drug Deliv Rev. 2018;132:81–103.
Liu LJ, Schlesinger M. MRI contrast agent concentration and tumor interstitial fluid pressure. J Theor Biol. 2016;406:52–60.
Zhan W, Wang CH. Convection enhanced delivery of chemotherapeutic drugs into brain tumour. J Control Release Off J Control Release Soci. 2018;271:74–87.
Yao W, Li Y, Ding G. Interstitial fluid flow: the mechanical environment of cells and foundation of meridians. Evid Based Complement Alternat Med. 2012;2012:853516.
Thomsen M, Hernandez-Garcia A, Mathiesen J, Poulsen M, Sørensen DN, Tarnow L, et al. Model study of the pressure build-up during subcutaneous injection. PLoS One. 2014;9(8):e104054.
Nedjar B. Formulation of a nonlinear porosity law for fully saturated porous media at finite strains. J Mech Phys Solids. 2013;61:537–56.
Zhang H, Liu J, Elsworth D. How sorption-induced matrix deformation affects gas flow in coal seams: a new FE model. Int J Rock Mech Min Sci. 2008;45(8):1226–36.
Detournay E, Cheng AHD. 5 - fundamentals of poroelasticity. In: Fairhurst C, editor. Analysis and design methods: Pergamon; 1993. p. 113–71.
Hommel J, Coltman E, Class H. Porosity–permeability relations for evolving pore space: a review with a focus on (bio-)geochemically altered porous media. Transp Porous Media. 2018;124(2):589–629.
Bear J. Dynamics of fluids in porous media: Dover Publications; 2013.
Darcy H. Les fontaines publiques de la ville de Dijon: Exposition et application des principes à suivre et des formules à employer dans les questions de distribution d'eau : Ouvrage terminé par un appendice relatif aux fournitures d'eau de plusieurs villes, au filtrage des eaux et à la fabrication des tuyaux de fonte, de plomb, de tôle et de bitume: V. Dalmont; 1856.
Song H. Engineering fluid mechanics: Singapore : springer Singapore : imprint: springer; 2018.
Shrestha P, Stoeber B. Fluid absorption by skin tissue during intradermal injections through hollow microneedles. Sci Rep. 2018;8(1):13749.
Joodaki H, Panzer MB. Skin mechanical properties and modeling: a review. Proc Inst Mech Eng H J Eng Med. 2018;232(4):323–43.
Gallagher AJ, Annaidh AN, Bruyère K et al. Dynamic tensile properties of human skin. In.: International Research Council on the Biomechanics of Injury; 2012.
Støverud KH, Darcis M, Helmig R, Hassanizadeh SM. Modeling concentration distribution and deformation during convection-enhanced drug delivery into brain tissue. Transp Porous Media. 2012;92(1):119–43.
Dokos S. Modelling organs, tissues, cells and devices: using MATLAB and COMSOL multiphysics: springer Berlin Heidelberg; 2017.
Scallan J, Huxley VH, Korthuis RJ. Integrated systems physiology: from molecule to function to disease. In. Capillary fluid exchange: regulation, functions, and pathology. Morgan & Claypool Life Sciences; 2010.
Yuan Y, Chilian WM, Granger HJ, Zawieja DC. Permeability to albumin in isolated coronary venules. Am J Phys Heart Circ Phys. 1993;265(2):H543–52.
Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994;74(1):163–219.
Anderson OA, Jackson TL, Singh JK, Hussain AA, Marshall J. Human transscleral albumin permeability and the effect of topographical location and donor age. Invest Ophthalmol Vis Sci. 2008;49(9):4041–5.
COMSOL I. COMSOL Multiphysics Reference Manual, version 5.3a.
Yeh G-T. On the computation of Darcian velocity and mass balance in the finite element modeling of groundwater flow. Water Resour Res. 1981;17(5):1529–34.
Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1994;18(2):79–83.
Thomas LW. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cognate Med Sci. 1962;47(2):179–88.
Nicholson C. Diffusion and related transport mechanisms in brain tissue. Rep Prog Phys. 2001;64(7):815–84.
Jain RK. Transport of molecules in the tumor Interstitium: a review. Cancer Res. 1987;47(12):3039–51.
Kim H, Park H, Lee SJ. Effective method for drug injection into subcutaneous tissue. Sci Rep. 2017;7(1):9613.
Comley K, Fleck N. Deep penetration and liquid injection into adipose tissue. J Mech Mater Struct. 2011;6:127–40.
Wang W, Guo X, Shen G, Bai G, Wei Z, Liu J, et al. Skin and subcutaneous tissue thickness at insulin injection sites in Chinese diabetes patients: clinical implications. Diabetes Metab. 2016;42(5):374–7.
Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–30.
Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J Physiol. 2011;589(12):2935–43.
Goh Y-MF, Kong HL, Wang C-H. Simulation of the delivery of doxorubicin to Hepatoma. Pharm Res. 2001;18(6):761–70.
Rippe B, Kamiya A, Folkow B. Simultaneous measurements of capillary diffusion and filtration exchange during shifts in filtration-absorption and at graded alterations in the capillary permeability surface area products (PS). Acta Physiol Scand. 1978;104(3):318–36.
Shields JD, Borsetti M, Rigby H, Harper SJ, Mortimer PS, Levick JR, et al. Lymphatic density and metastatic spread in human malignant melanoma. Br J Cancer. 2004;90(3):693–700.
Platt AM, Randolph GJ. Chapter two - dendritic cell migration through the lymphatic vasculature to lymph nodes. In: Murphy KM, Merad M, editors. Advances in Immunology: Academic Press; 2013. p 51–68.
Schaefer B, Bartosova M, Macher-Goeppinger S, Ujszaszi A, Wallwiener M, Nyarangi-Dix J, et al. Quantitative Histomorphometry of the healthy peritoneum. Sci Rep. 2016;6:21344.
Spiegel M, Vesti B, Shore A, Franzeck UK, Becker F, Bollinger A. Pressure of lymphatic capillaries in human skin. Am J Phys Heart Circ Phys. 1992;262(4):H1208–10.
Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M, Gradalski T. Tissue fluid pressure and flow in the subcutaneous tissue in lymphedema - hints for manual and pneumatic compression therapy. Phlebolymphology. 2010;17:144–50.
Li C, Guan G, Reif R, Huang Z, Wang RK. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J R Soc Interface. 2012;9(70):831–41.
Rafsanjani A, Derome D, Carmeliet J. Poromechanical modeling of moisture induced swelling anisotropy in cellular tissues of softwoods. RSC Adv. 2015;5(5):3560–6.
Dehghani H, Noll I, Penta R, Menzel A, Merodio J. The role of microscale solid matrix compressibility on the mechanical behaviour of poroelastic materials. Eur J Mech A Solids. 2020;83:103996.
McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89–96.
Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9.
Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol. 2010;588(Pt 1):243–54.
Karlsson D, Zacchi G, Axelsson A. Electronic speckle pattern interferometry: a tool for determining diffusion and partition coefficients for proteins in gels. Biotechnol Prog. 2002;18(6):1423–30.
Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in Normal and neoplastic tissues by fluorescence Photobleaching. Proc Natl Acad Sci U S A. 1989;86(14):5385–9.
Clauss MA, Jain RK. Interstitial transport of rabbit and sheep antibodies in normal and neoplastic tissues. Cancer Res. 1990;50(12):3487–92.
Siflinger-Birnboim A, del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132(1):111–7.
Zadro R, Pokric B, Pucar Z. Dependence of diffusion coefficients and immunoprecipitating titers on pH: human serum transferrin, immunoglobulin a, human chorionic somatomammotropin, and their rabbit antibodies. Anal Biochem. 1981;117(2):238–44.
Wasilewska M, Adamczyk Z, Pomorska A, Nattich-Rak M, Sadowska M. Human serum albumin adsorption kinetics on silica: influence of protein solution stability. Langmuir ACS J Surf Colloids. 2019;35(7):2639–48.
Cannistraro S, Sacchetti F. Rotational and translational dynamics of human albumin. Phys Rev A. 1986;33(1):745–6.
Monkos K. Temperature and concentration dependence of translational diffusion coefficient for human serum albumin in aqueous solutions at different pH. Ann Acad Med Silesiensis. 2013;67:184–93.
Aragon S, Hahn DK. Precise boundary element computation of protein transport properties: diffusion tensors, specific volume, and hydration. Biophys J. 2006;91(5):1591–603.
Charlwood PA. Sedimentation and diffusion of human albumins. I. Normal human albumins at a low concentration. Biochem J. 1952;51(1):113–8.
Oncley JL, Scatchard G, Brown A. Physical-chemical characteristics of certain of the proteins of normal human plasma. J Phys Colloid Chem. 1947;51(1):184–98.
Medda L, Monduzzi M, Salis A. The molecular motion of bovine serum albumin under physiological conditions is ion specific; 2015.
Kihara T, Ito J, Miyake J. Measurement of biomolecular diffusion in extracellular matrix condensed by fibroblasts using fluorescence correlation spectroscopy. PLoS One. 2013;8(11):e82382.
Varghese LT, Sinha RK, Irudayaraj J. Study of binding and denaturation dynamics of IgG and anti-IgG using dual color fluorescence correlation spectroscopy. Anal Chim Acta. 2008;625(1):103–9.
Reitan NK, Juthajan A, Lindmo T, Davies CDL. Macromolecular diffusion in the extracellular matrix measured by fluorescence correlation spectroscopy: SPIE; 2008.
Pokrić B, Pučar Z. The two-cross immunodiffusion technique: diffusion coefficients and precipitating titers of IgG in human serum and rabbit serum antibodies. Anal Biochem. 1979;93:103–14.
Jøssang T, Feder J, Rosenqvist E. Photon correlation spectroscopy of human IgG. J Protein Chem. 1988;7(2):165–71.
Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66(2 Pt 1):508–15.
Brandt JP, Patapoff TW, Aragon SR. Construction, MD simulation, and hydrodynamic validation of an all-atom model of a monoclonal IgG antibody. Biophys J. 2010;99(3):905–13.
el-Kareh AW, Braunstein SL, Secomb TW. Effect of cell arrangement and interstitial volume fraction on the diffusivity of monoclonal antibodies in tissue. Biophys J. 1993;64(5):1638–46.
Smith L, Andreasson S, Thoren-Tolling K, Rippe B, Risberg B. Sepsis in sheep reduces pulmonary microvascular sieving capacity. J Appl Physiol. 1987;62(4):1422–9.
Hollander W, Reilly P, Burrows BA. Lymphatic flow in human subjects as indicated by the disappearance of 1-131-labeled albumin from the subcutaneous tissue. J Clin Invest. 1961;40(2):222–33.
Hays MT, McGuire RA. Distribution of subcutaneous thyroxine, triiodothyronine, and albumin in man: comparison with intravenous administration using a kinetic model. J Clin Endocrinol Metab. 1980;51(5):1112–7.
Pain SJ, Nicholas RS, Barber RW, Ballinger JR, Purushotham AD, Mortimer PS, et al. Quantification of lymphatic function for investigation of lymphedema: depot clearance and rate of appearance of soluble macromolecules in blood. J Nucl Med Off Publ Soc Nucl Med. 2002;43(3):318–24.
Stanton AW, Modi S, Mellor RH, Peters AM, Svensson WE, Levick JR, et al. A quantitative lymphoscintigraphic evaluation of lymphatic function in the swollen hands of women with lymphoedema following breast cancer treatment. Clinical science (London, England : 1979). 2006;110(5):553–61.
Stanton AW, Svensson WE, Mellor RH, Peters AM, Levick JR, Mortimer PS. Differences in lymph drainage between swollen and non-swollen regions in arms with breast-cancer-related lymphoedema. Clinical science (London, England : 1979). 2001;101(2):131–40.
Pain SJ, Barber RW, Ballinger JR, Solanki CK, Mortimer PS, Purushotham AD, et al. Local vascular access of radioprotein injected subcutaneously in healthy subjects and patients with breast cancer-related lymphedema. J Nucl Med Off Publ Soc Nucl Med. 2004;45(5):789–96.
Pain SJ, Barber RW, Solanki CK, Ballinger JR, Britton TB, Mortimer PS, et al. Short-term effects of axillary lymph node clearance surgery on lymphatic physiology of the arm in breast cancer. J Appl Physiol. 2005;99(6):2345–51.
O'Mahony S, Solanki CK, Barber RW, Mortimer PS, Purushotham AD, Peters AM. Imaging of lymphatic vessels in breast cancer-related lymphedema: intradermal versus subcutaneous injection of 99mTc-immunoglobulin. AJR Am J Roentgenol. 2006;186(5):1349–55.
Collins DS, Kourtis LC, Thyagarajapuram NR, Sirkar R, Kapur S, Harrison MW, et al. Optimizing the bioavailability of subcutaneously administered biotherapeutics through Mechanochemical drivers. Pharm Res. 2017;34(10):2000–11.
Nugent LJ, Jain RK. Plasma pharmacokinetics and interstitial diffusion of macromolecules in a capillary bed. Am J Phys Heart Circ Phys. 1984;246(1):H129–37.
Fox JR, Wayland H. Interstitial diffusion of macromolecules in the rat mesentery. Microvasc Res. 1979;18(2):255–76.
Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473–84.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, F., Hou, P., Corpstein, C.D. et al. Multiphysics Modeling and Simulation of Subcutaneous Injection and Absorption of Biotherapeutics: Model Development. Pharm Res 38, 607–624 (2021). https://doi.org/10.1007/s11095-021-03032-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03032-w