ABSTRACT
Purpose
To evaluate the impact of curcumin on the disposition of resveratrol phase II metabolites in vivo, and explain the observations by performing in vitro studies in transporter-overexpressed cells.
Methods
Pharmacokinetic studies of resveratrol with and without the co-administration of curcumin were performed in both FVB wild-type and Bcrp1 (−/−) mice. Human UGT1A9-overexpressing HeLa cells and human MRP2-overexpressing MDCK II-UGT1A1 cells were used as in vitro tools to further determine the impact of curcumin as a transporter inhibitor on resveratrol metabolites.
Results
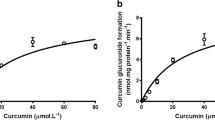
We observed higher exposure of resveratrol conjugates in Bcrp1 (−/−) mice compared to wild-type mice. In wild-type mice, curcumin increased the AUC of resveratrol glucuronide by 4-fold compared to the mice treated without curcumin. The plasma levels of resveratrol and its sulfate conjugate also increased moderately. In Bcrp1 (−/−) mice, there was a further increase (6-fold increase) in AUC of resveratrol glucuronide observed when curcumin was co-administered compared to AUC values obtained in wild-type mice without curcumin treatment. In the presence of 50 nM curcumin, the clearance of resveratrol-3-O-glucuronide and resveratrol-3-O-sulfate reduced in both MRP2-overexpressing MDCKII-UGT1A1 cells and Human UGT1A9-overexpressing HeLa cells.
Conclusions
These results suggest that curcumin alters the phase II distribution of resveratrol through inhibiting efflux transporters including MRP2 and BCRP.






Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- AUC:
-
Area under the plasma concentration curve
- BCRP/Bcrp:
-
Breast cancer resistance protein
- HBSS:
-
Hanks’ balanced salt solution
- MRP:
-
Multidrug resistance-associated protein
- MS/MS:
-
Tandem mass spectrometry
- N.A.:
-
Not available.
- PAPS:
-
β-glucuronidases, sulfatase, 3′-Phosphoadenosine 5′-phosphosulfate
- RES-3-O-G:
-
Resveratrol-3-O-glucuronide
- RES-3-O-S:
-
Resveratrol-3-O-sulfate
- RES-4′-O-G:
-
Resveratrol-4′-O-glucuronide
- RES-4′-O-S:
-
Resveratrol-4′-O-sulfate
- SULT:
-
Sulfotransferase
- UDPGA:
-
Uridine diphosphoglucuronic acid
- UGT:
-
UDP-glucuronosyltransferase
- UPLC:
-
Ultraperformance liquid chromatography
- WT:
-
Wild type
REFERENCES
Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50:431–5.
Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–85.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42.
Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–4.
Bhatand KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210–29.
Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–19.
Schouten A, Wagemakers L, Stefanato FL, van der Kaaij RM, van Kan JA. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Mol Microbiol. 2002;43:883–94.
Luand R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179:297–304.
Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4 T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–5.
Berge G, Ovrebo S, Eilertsen E, Haugen A, Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br J Cancer. 2004;91:1380–3.
Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–81.
Gao X, Deeb D, Media J, Divine G, Jiang H, Chapman RA, et al. Immunomodulatory activity of resveratrol: discrepant in vitro and in vivo immunological effects. Biochem Pharmacol. 2003;66:2427–35.
Walle T, Hsieh F, DeLegge MH, Oatis Jr JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82.
Baurand JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506.
Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52.
Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136:57–66.
Polycarpou E, Meira LB, Carrington S, Tyrrell E, Modjtahedi H, Carew MA. Resveratrol 3-O-D-glucuronide and resveratrol 4′-O-D-glucuronide inhibit colon cancer cell growth: evidence for a role of A3 adenosine receptors, cyclin D1 depletion, and G1 cell cycle arrest. Mol Nutr Food Res. 2013;57:1708–17.
Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, et al. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–43.
Zunino SJ, Storms DH, Newman JW, Pedersen TL, Keen CL, Ducore JM. Dietary resveratrol does not delay engraftment, sensitize to vincristine or inhibit growth of high-risk acute lymphoblastic leukemia cells in NOD/SCID mice. Int J Oncol. 2012;41:2207–12.
Islam MA, Bekele R, Vanden Berg JH, Kuswanti Y, Thapa O, Soltani S, et al. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol in Vitro. 2015;29:706–15.
Maier-Salamon A, Hagenauer B, Reznicek G, Szekeres T, Thalhammer T, Jager W. Metabolism and disposition of resveratrol in the isolated perfused rat liver: role of Mrp2 in the biliary excretion of glucuronides. J Pharm Sci. 2008;97:1615–28.
van de Wetering K, Burkon A, Feddema W, Bot A, de Jonge H, Somoza V, et al. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;75:876–85.
Maier-Salamon A, Bohmdorfer M, Riha J, Thalhammer T, Szekeres T, Jaeger W. Interplay between metabolism and transport of resveratrol. Ann N Y Acad Sci. 2013;1290:98–106.
International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36.
Keppler D. Uptake and efflux transporters for conjugates in human hepatocytes. Methods Enzymol. 2005;400:531–42.
Mizuno N, Takahashi T, Kusuhara H, Schuetz JD, Niwa T, Sugiyama Y. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one). Drug Metab Dispos. 2007;35:2045–52.
Alvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomas-Barberan F, et al. Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice. Drug Metab Dispos. 2011;39:2008–12.
Yang Z, Zhu W, Gao S, Yin T, Jiang W, Hu M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:1883–93.
Alfaras I, Perez M, Juan ME, Merino G, Prieto JG, Planas JM, et al. Involvement of breast cancer resistance protein (BCRP1/ABCG2) in the bioavailability and tissue distribution of trans-resveratrol in knockout mice. J Agric Food Chem. 2010;58:4523–8.
Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305.
Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–71.
Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006.
Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68:2043–52.
Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother Pharmacol. 2006;57:376–88.
Wortelboer HM, Usta M, van Zanden JJ, van Bladeren PJ, Rietjens IM, Cnubben NH. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of alpha, beta-unsaturated carbonyl compounds. Biochem Pharmacol. 2005;69:1879–90.
Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480–7.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9.
Jiang W, Xu B, Wu B, Yu R, Hu M. UDP-glucuronosyltransferase (UGT) 1A9-overexpressing HeLa cells is an appropriate tool to delineate the kinetic interplay between breast cancer resistance protein (BRCP) and UGT and to rapidly identify the glucuronide substrates of BCRP. Drug Metab Dispos. 2012;40:336–45.
Zhu W, Xu H, Wang SW, Hu M. Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. AAPS J. 2010;12:525–36.
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–9.
Volak LP, Ghirmai S, Cashman JR, Court MH. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. 2008;36:1594–605.
Vietri M, Pietrabissa A, Mosca F, Spisni R, Pacifici GM. Curcumin is a potent inhibitor of phenol sulfotransferase (SULT1A1) in human liver and extrahepatic tissues. Xenobiotica. 2003;33:357–63.
Wortelboer HM, Usta M, van der Velde AE, Boersma MG, Spenkelink B, van Zanden JJ, et al. Interplay between MRP inhibition and metabolism of MRP inhibitors: the case of curcumin. Chem Res Toxicol. 2003;16:1642–51.
Abe Y, Fujiwara R, Oda S, Yokoi T, Nakajima M. Interpretation of the effects of protein kinase C inhibitors on human UDP-glucuronosyltransferase 1A (UGT1A) proteins in cellulo. Drug Metab Pharmacokinet. 2011;26:256–65.
Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem. 2008;283:23048–61.
Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, et al. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–76.
Frozza RL, Bernardi A, Paese K, Hoppe JB, da Silva T, Battastini AM, et al. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol. 2010;6:694–703.
Kusuhara H, Furuie H, Inano A, Sunagawa A, Yamada S, Wu C, et al. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br J Pharmacol. 2012;166:1793–803.
Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–94.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63.
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:183–9.
Brand W, Oosterhuis B, Krajcsi P, Barron D, Dionisi F, van Bladeren PJ, et al. Interaction of hesperetin glucuronide conjugates with human BCRP, MRP2 and MRP3 as detected in membrane vesicles of overexpressing baculovirus-infected Sf9 cells. Biopharm Drug Dispos. 2011;32:530–5.
Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J Pharmacol Exp Ther. 2004;309:156–64.
Kitamura Y, Kusuhara H, Sugiyama Y. Functional characterization of multidrug resistance-associated protein 3 (mrp3/abcc3) in the basolateral efflux of glucuronide conjugates in the mouse small intestine. J Pharmacol Exp Ther. 2010;332:659–66.
Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, et al. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther. 2006;319:1485–91.
Guo A, Marinaro W, Hu P, Sinko PJ. Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT). Drug Metab Dispos. 2002;30:457–63.
Brill SS, Furimsky AM, Ho MN, Furniss MJ, Li Y, Green AG, et al. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J Pharm Pharmacol. 2006;58:469–79.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a grant from the National Institutes of Health (GM070737) to MH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
(DOCX 378 kb)
Rights and permissions
About this article
Cite this article
Ge, S., Yin, T., Xu, B. et al. Curcumin Affects Phase II Disposition of Resveratrol Through Inhibiting Efflux Transporters MRP2 and BCRP. Pharm Res 33, 590–602 (2016). https://doi.org/10.1007/s11095-015-1812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1812-1