ABSTRACT
Purpose
Antimicrobial preservatives are known to interact with proteins and potentially affect their stability in aqueous solutions. In this systematic study, the interactions of a model peptide with three commonly used preservatives, benzyl alcohol, phenol and m-cresol, were evaluated.
Methods
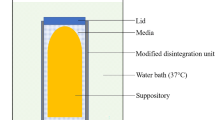
The impact on peptide oligomerization was studied using GC-MALS, SEC-MALS and DLS, antimicrobial efficiency of different formulations were studied using the Ph. Eur. antimicrobial efficacy test, and the molecular adsorption of preservative molecules on reversible peptide oligomers was monitored using NMR.
Results
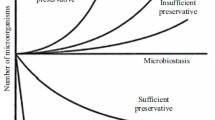
The hydrodynamic radius and molar mass of the peptide oligomers was shown to clearly increase in the presence of m-cresol but less significantly with phenol and benzyl alcohol. The increase in size was most likely caused by peptide self-interactions becoming more attractive, leading to reversible oligomerization. On the other hand, increasing the concentration of peptide in multi-dose formulations led to reduced molecular mobility and decreased antimicrobial efficacy of all preservatives.
Conclusions
Peptide-preservative interactions not only affect peptide self-interactions, but also antimicrobial efficiency of the preservatives and are thus of significant relevance. Adsorption of preservatives on oligomeric states of peptides is proposed as a mechanism to explain this reduced antimicrobial efficacy.





Similar content being viewed by others
Abbreviations
- \( {\overline{\mathrm{M}}}_{\mathrm{w}} \) :
-
Weight average molar mass
- \( {\overline{\mathrm{r}}}_{{}_{\mathrm{H}}} \) :
-
Hydrodynamic radius
- A2 :
-
Second virial coefficient
- AET:
-
Antimicrobial efficacy testing
- C0 :
-
Preservative concentration required to kill/inactivate all microbes in the absence of peptide
- CFU:
-
Colony forming unit
- CG-MALS:
-
Composition-gradient multi-angle light scattering
- Ci :
-
Preservative concentration required to kill/inactivate all microbes in the presence of peptide
- Dfree :
-
Preservative diffusion coefficient in the absence of peptide
- DLS:
-
Dynamic light scattering
- Dobs :
-
Preservative diffusion coefficient in the presence of peptide
- DOSY:
-
Diffusion ordered NMR spectroscopy
- Dpep :
-
Peptide diffusion coefficient
- f:
-
Fraction of live microbes after incubation
- fs :
-
Sequestered preservative fraction (in AET experiments)
- Mw :
-
Apparent molar mass
- NMR:
-
Nuclear magnetic resonance
- pI:
-
Isoelectric point
- PO/W :
-
Octanol-water partition coefficient
- Ppep :
-
Peptide-bound preservative fraction (in NMR experiments)
- SEC-MALS:
-
Size-exclusion chromatography, coupled with multi-angle light scattering detector
REFERENCES
United States Pharmacopoeia, USP <51>Antimicrobial effectiveness testing. Rockville.
European Pharmacopoeia, Ph. Eur. <5.1.3.> Efficacy of antimicrobial preservatives. Strasbourg.
Japanese Pharmacopoeia, JP <19> Preservative-effectiveness tests. Tokyo.
Meyer B, Ni A, Binghua H, Shi L. Antimicrobial preservative use in parenteral products: past and present. J Pharm Sci. 2007;96(12):3155–67.
Lam X, Patapoff T, Nguyen T. The effect of benzyl alcohol on recombinant human interferon-γ. Pharm Res. 1997;14(6):725–9.
Gupta S, Kaiseva E. Development of a multidose formulation for a humanized monoclonal antibody using experimental design techniques. AAPS Pharm Sci. 2003;5(8):1–9.
Bis R, Mallela K. Antimicrobial preservatives induce aggregation of interferon alpha-2a: the order in which preservatives induce protein aggregation is independent of the protein. Int J Pharm. 2014;472:356–61.
Wolff T, Youngman R. Oxidative metabolism of phenols: conversion of bromochatechol to protein binding species by O2 −. In: Bors W, Saran M, Tait D, editors. Oxygen radicals in chemistry and biology. Berlin: Walter de Gruyter and Co; 1984. p. 165–70.
Maa Y-F, Hsu C. Aggregation of recombinant human growth hormone induced by phenolic compounds. Int J Pharm. 1996;140:155–68.
Hutchings R, Singh S, Cabello-Villegas J, Mallela K. Effect of antimicrobial preservatives on partial protein unfolding and aggregation. J Pharm Sci. 2013;102(2):365–76.
Whittingham J, Edwards D, Antson A, Clarkson J, Dodson G. Interactions of phenol and m-cresol in the insulin hexamer, and their effects on the association properties of B28 Pro → Asp insulin analogues. Biochem. 1998;37(33):11516–23.
Swegat W, Schlitter J, Krüger P, Wollmer A. MD simulation of protein-ligand interaction: formation and dissociation of an insulin-phenol complex. Biophys J. 2003;84:1493–506.
Alford J, Fowler A, Wuttke D, Kerwin B, Latypov R, Carpenter J, et al. Effect of benzyl alcohol on recombinant human interleukin-1 receptor antagonist structure and hydrogen-deuterium exchange. J Pharm Sci. 2011;100(10):4215–24.
Loomis W. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31(A):528–44.
Roy S, Katayama D, Dong A, Kerwin B, Randolph T, Carpenter J. Temperature dependence of benzyl alcohol- and 8-anilinonaphtalene-1-sulfonate-induced aggregation of recombinant human interleukin-1 receptor antagonist. Biochem. 2006;45(12):3898–911.
Rahuel-Clermont S, French C, Kaarsholm N, Dunn M, Chou C. Mechanisms of stabilization of the insulin hexamer through allosteric ligand interactions. Biochem. 1997;36(19):5837–45.
Goyal M, Roy I, Banerjee U, Sharma V, Bansal A. Role of benzyl alcohol in the prevention of heat-induced aggregation and inactivation of hen egg lysozyme. Eur J Pharm Biopharm. 2009;71:367–76.
Goyal M, Roy I, Amin A, Banerjee U, Bansal A. Stabilization of lysozyme by benzyl alcohol: surface tension and thermodynamic parameters. J Pharm Sci. 2010;99(10):4149–61.
Some D, Kendrick S. Characterization of protein-protein interactions via static and dynamic light scattering. In: Cai J, Wang R, editors. Protein interactions. Vienna: InTech; 2013. p. 401–26.
Attri A, Minton A. New methods for measuring macromolecular interactions in solution via static light scattering: basic methodology and application to nonassociating and self-associating proteins. Anal Biochem. 2005;337:103–10.
Wu D, Chen A, Johnson C. An Improved diffusion-ordered spectroscopy experiment incorporating bipolar-gradient pulses. J Magn Reson A. 1995;115:260–4.
Stejskal E, Tanner J. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–92.
Strøm A, Kaasen I. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol Microbiol. 1993;8(2):205–10.
Wilson J, Lyall J, McBride R, Murray J, Smith G. Partition coefficients of some aromatic alcohols in an n-heptane/water system and their relationship to minimum inhibitory concentration against pseudomonas aeruginosa and staphylococcus aureus. J Clin Hosp Pharm. 1981;6:63–6.
Sangster J. Octanol – water partition coefficients of simple organic compounds. J Phys Chem Ref Data. 1989;13(3):1111–227.
Pisano F, Kostenbauder H. Interaction of preservatives with macromolecules II: correlation of binding data with required preservative concentrations of p-hydroxybenzoates in the presence of Tween 80. J Am Pharm Assoc. 1957;48(6):310–4.
Blanchard J, Fink W, Duffy J. Effect of sorbitol on interaction of phenolic preservatives with polysorbate 80. J Pharm Sci. 1977;66(10):1470–3.
Pinholt C, Kapp S, Bukrinsky J, Hostrup S, Frokjaer S, Norde W, et al. Influence of acylation on the adsorption of GLP-2 to hydrophobic surfaces. Int J Pharm. 2013;440:63–71.
Hagerman A, Butler L. The specificity of proanthocyanidin-protein interactions. J Biolog Chem. 1981;256(9):4494–7.
Charlton A, Baxter N, Khan M, Moir A, Haslam E, Davies A, et al. Polyphenol/peptide binding and precipitation. J Agric Food Chem. 2002;50:1593–601.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors wish to acknowledge Claudia Bleyer and Olivier Ortschitt (F. Hoffmann – La Roche Ltd.) for their work with preservative content analyses, Dr. Arne Rufer for his work with AUC analyses, Dr. Sonoko Kanai and Michaela Grass (F. Hoffmann – La Roche Ltd.) for participating in the light scattering experiments and Jenny Train and Anita De Vivo (F. Hoffmann – La Roche Ltd.) for their assistance in manufacturing the sterile formulations for antimicrobial efficacy studies. Furthermore, Dr. Kishore Ravuri (F. Hoffmann – La Roche Ltd.) is acknowledged for his participation in experimental planning and Dr. Sulabh Patel and Tobias Werk (F. Hoffmann – La Roche Ltd.) for their input in interpreting the AET results. Finally, we would also like to acknowledge Prof. Jörg Huwyler (University of Basel, Switzerland) for reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
The Stejskal-Tanner type plots (22) below show the logarithm of spectral amplitudes in DOSY type spectra [ln(I/I0)], which are displayed in dependence of the square of the gradient (G2). Diffusion constants were fitted by use of the analysis module included in the NMR software package Topspin (Bruker, Fällanden, Switzerland). Ratios of diffusion constants for excipients in presence and absence of peptides (shown in the plots) were used to estimate adsorbed fractions of the excipients on the peptide or oligomers thereof according to Eqs. 4–6. The graphs in black show the measurements of 5 mg/ml preservative in the absence of peptide, and the gray ones the measurements of 5 mg/ml preservative with 10 mg/ml peptide. (GIF 6259 kb)
Rights and permissions
About this article
Cite this article
Heljo, P., Ross, A., Zarraga, I.E. et al. Interactions Between Peptide and Preservatives: Effects on Peptide Self-Interactions and Antimicrobial Efficiency In Aqueous Multi-Dose Formulations. Pharm Res 32, 3201–3212 (2015). https://doi.org/10.1007/s11095-015-1697-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1697-z