Abstract
Purpose
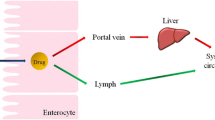
Intestinal lymphatic transport of specific lipophilic drugs offers therapeutic advantages and maximises oral bioavailability. The aims of this study were; to compare intestinal lymphatic transport of a range of drugs and to investigate the influence of cyclosporine A on the mechanism/extent of lymphatic transport.
Methods
Caco2 cells and an anaesthetised mesenteric lymphatic cannulated rat model were used for in vitro and in vivo studies. Lymphatic transport of three lipophilic drugs was directly compared in a long chain fatty acid formulation. In addition, the impact of cyclosporine A on triglyceride turnover was evaluated in vivo and in vitro.
Results
The extent of intestinal lymphatic transport in rats was positively correlated with drug solubility in triglyceride and negatively correlated with drug aqueous solubility. Cyclosporine A displayed non-linear lymphatic transport kinetics and reduced intestinal lymph triglyceride. In vitro experiments indicated that the cellular processes affected were intracellular lipid processing and/or lipid secretion.
Conclusions
The linear correlations obtained using a range of lipophilic drugs confirm that the simplified approach of determining aqueous or triglyceride drug solubility is useful in predicting the extent of lymphatic transport. In vitro experiments correlated with in vivo observations, demonstrating the usefulness of the Caco-2 model for mechanistic investigations.





Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DDT:
-
Dichlorodiphenyltrichloroethane
- FA:
-
Fatty acids
- FaSSIF:
-
Fasted simulated state intestinal fluid
- HPLC:
-
High performance liquid chromatography
- LCFA:
-
Long chain fatty acids
- LCT:
-
Long chain triglyceride
- LOQ:
-
Limit of quantitation
- PBS:
-
Phosphate buffered saline
- P-gp:
-
P-glycoprotein
- TG:
-
Triglyceride
- TLC:
-
Thin layer chromatography
- TRL:
-
Triglyceride rich lipoproteins
References
Porter CJH, Williams HD, Trevaskis NL. Recent advances in lipid-based formulation technology. Pharm Res. 2013;30(12):2971–5.
O’Driscoll CM, Griffin BT. Biopharmaceutical challenges associated with drugs with low aqueous solubility - the potential impact of lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):617–24.
O’Reilly JR, Corrigan OI, O’Driscoll CM. The effect of mixed micellar systems, bile-salt fatty-acids, on the solubility and intestinal-absorption of clofazimine (B663) in the anaesthetized rat. Int J Pharm. 1994;109(2):147–54.
O’Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15(5):405–15.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Trevaskis NL, Charman WN, Porter CJH. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–16.
Trevaskis NL, Charman WN, Porter CJH. Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation? Mol Pharm. 2010;7(6):2297–309.
Griffin BT, O’Driscoll CM. An examination of the effect of intestinal first pass extraction on intestinal lymphatic transport of saquinavir in the rat. Pharm Res. 2008;25(5):1125–33.
Charman WNA, Stella VJ. Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm. 1986;34(1–2):175–8.
Myers RA, Stella VJ. Factors affecting the lymphatic transport of penclomedine (NSC-338720), a lipophilic cytotoxic drug - comparison to DDT and hexachlorobenzene. Int J Pharm. 1992;80(1):51–62.
Porter CJH, Charman SA, Charman WN. Lymphatic transport of halofantrine in the triple-cannulated anesthetized rat model: effect of lipid vehicle dispersion. J Pharm Sci. 1996;85(4):351–6.
Hauss DJ, Mehta SC, Radebaugh GW. Targeted lymphatic transport and modified systemic distribution of CI-976, a lipophilic lipid-regulator drug, via a formulation approach. Int J Pharm. 1994;108(2):85–93.
Caliph SM, Charman WN, Porter CJH. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 2000;89(8):1073–84.
Faisal W, O’Driscoll CM, Griffin BT. Bioavailability of lycopene in the rat: the role of intestinal lymphatic transport. J Pharm Pharmacol. 2010;62(3):323–31.
Nankervis R, Davis SS, Day NH, Shaw PN. Intestinal lymphatic transport of three retinoids in the rat after oral administration: Effect of lipophilicity and lipid vehicle. Int J Pharm. 1996;130(1):57–64.
Griffin BT, O’Driscoll CM. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol. 2006;58(7):917–25.
Seeballuck F, Ashford MB, O’Driscoll CM. The effects of Pluronic (R) block copolymers and Cremophor (R) EL on intestinal lipoprotein processing and the potential link with P-glycoprotein in Caco-2 cells. Pharm Res. 2003;20(7):1085–92.
Seeballuck F, Lawless E, Ashford MB, O’Driscoll CM. Stimulation of triglyceride-rich lipoprotein secretion by polysorbate 80: in vitro and in vivo correlation using Caco-2 cells and a cannulated rat intestinal lymphatic model. Pharm Res. 2004;21(12):2320–6.
Khoo SM, Edwards GA, Porter CJH, Charman WN. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J Pharm Sci. 2001;90(10):1599–607.
Khoo SM, Prankerd RJ, Edwards GA, Porter CJH, Charman WN. A physicochemical basis for the extensive intestinal lymphatic transport of a poorly lipid soluble antimalarial, halofantrine hydrochloride, after postprandial administration to dogs. J Pharm Sci. 2002;91(3):647–59.
Trevaskis NL, Caliph SM, Nguyen G, Tso P, Charman WN, Porter CJH. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm Res. 2013;30(12):3254–70.
Noguchi T, Charman WNA, Stella VJ. Lymphatic appearance of DDT in thoracic or mesenteric lymph duct cannulated rats. Int J Pharm. 1985;24(2–3):185–92.
Dahan A, Mendelman A, Amsili S, Ezov N, Hoffman A. The effect of general anesthesia on the intestinal lymphatic transport of lipophilic drugs: Comparison between anesthetized and freely moving conscious rat models. Eur J Pharm Sci. 2007;32(4–5):367–74.
Charman WNA, Stella VJ. Effects of lipid class and lipid vehicle volume on the intestinal lymphatic transport of DDT. Int J Pharm. 1986;33(1–3):165–72.
Gershkovich P, Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci. 2005;26(5):394–404.
Holm R, Hoest J. Successful in silico predicting of intestinal lymphatic transfer. Int J Pharm. 2004;272(1–2):189–93.
O’Driscoll CM, Myers RA, Stella VJ. Blood and lymph transport of DDT after oral and parenteral administration to anesthetized rats. Int J Pharm. 1991;73(2):177–83.
Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509.
Gibney MJ, Connolly A. Uptake of exogenous and endogenous eicosapentaenoic acid by cultured human mononuclear cells. Br J Nutr. 1988;60(1):13–20.
Field FJ, Born E, Chen H, Murthy S, Mathur SN. Esterification of plasma membrane cholesterol and triacylglycerol-rich lipoprotein secretion in CaCo-2 cells: possible role of p-glycoprotein. J Lipid Res. 1995;36(7):1533–43.
Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56(9):1315–34.
Dahan A, Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24:381–8.
Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807–18.
Ueda CT, Lemaire M, Gsell G, Nussbaumer K. Intestinal lymphatic absorption of cyclosporin A following oral administration in an olive oil solution in rats. Biopharm Drug Dispos. 1983;4(2):113–24.
Takada K, Yoshimura H, Shibata N, Masuda Y, Yoshikawa H, Muranishi S, et al. Effect of administration route on the selective lymphatic delivery of cyclosporine A by lipid-surfactant mixed micelles. J Pharmacobio Dyn. 1986;9(2):156–60.
Wang TY, Liu M, Portincasa P, Wang DQH. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Investig. 2013;43(11):1203–23.
Ismailos G, Reppas C, Dressman JB, Macheras P. Unusual solubility behaviour of cyclosporin A in aqueous media. J Pharm Pharmacol. 1991;43(4):287–9.
Patton JS, Stone B, Papa C, Abramowitz R, Yalkowsky S. Solubility of fatty acids and other hydrophobic molecules in liquid trioleoylglycerol. J Lipid Res. 1984;25(2):189–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawless, E., Griffin, B.T., O’Mahony, A. et al. Exploring the Impact of Drug Properties on the Extent of Intestinal Lymphatic Transport - In Vitro and In Vivo Studies. Pharm Res 32, 1817–1829 (2015). https://doi.org/10.1007/s11095-014-1578-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1578-x