Abstract
Purpose
To develop and evaluate molecularly imprinted nanocarriers for sustained release of erythromycin in physiological buffer media.
Methods
Erythromycin-imprinted poly(methacrylic acid–co–trimethylolpropane trimethacrylate) nanocarriers and corresponding control nanocarriers were prepared by free-radical precipitation polymerization. The nanocarriers were characterized by transmission electron microscopy, dynamic light scattering, and nitrogen sorption analysis. Binding studies were carried out with erythromycin and five structurally unrelated drugs. Molecular descriptors of the drugs were computed and correlated to measured binding data by multivariate data analysis. Loading with erythromycin and in vitro release studies were carried out in physiological buffer media. Kinetic models were fitted to drug release data.
Results
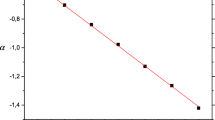
The template affected the size and morphology of the nanocarriers. Binding isotherms showed that erythromycin-imprinted nanocarriers had a higher erythromycin binding capacity than corresponding control nanocarriers. Multivariate data analysis, correlating binding to molecular descriptors of the drugs, indicated a molecular imprinting effect. Erythromycin loading capacity was 76 mg/g with a loading efficiency of 87%. Release studies in physiological buffer showed an initial burst release of a quarter of loaded erythromycin during the first day and an 82% release after a week. The release was best described by the Korsmeyer-Peppas model.
Conclusions
Sustained release of erythromycin in physiological buffer was demonstrated.











Similar content being viewed by others
Abbreviations
- AIBN:
-
2,2’-azobisisobutyronitrile
- BET:
-
Brunauer, Emmett, and Teller
- BJH:
-
Barrett, Joyner, and Halenda
- Boc:
-
Tert-butyloxycarbonyl
- DLS:
-
Dynamic light scattering
- EDMA:
-
Ethylene glycol dimethacrylate
- ERY:
-
Erythromycin
- HPLC:
-
High-performance liquid chromatography
- MAA:
-
Methacrylic acid
- MeOH:
-
Methanol
- MIP:
-
Molecularly imprinted polymer
- NIP:
-
Non-imprinted polymer
- PBS:
-
Phosphate buffered saline
- Phe:
-
Phenylalanine
- PLS:
-
Partial least square
- PRESS:
-
Predicted residual sums of squares
- RSS:
-
Residual sum of squares
- SPE:
-
Solid-phase extraction
- TEM:
-
Transmission electron microscope
- TFA:
-
Trifluoroacetic acid
- TRIM:
-
Trimethylolpropane trimethacrylate
- UV:
-
Ultraviolet
References
Xu Z-Q, Flavin MT, Eiznhamer DA. Macrolides and ketolides. In: Dougherty TJ, Pucci MJ, editors. Antibiotic Discovery and Development. New York: Springer; 2012. p. 181–228.
Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615.
Fumimori T, Honda S, Migita K, Hamada M, Yoshimuta T, Honda J, et al. Erythromycin suppresses the expression of cyclooxygenase-2 in rheumatoid synovial cells. J Rheumatol. 2004;31(3):436–41.
Ren W, Blasier R, Peng X, Shi T, Wooley PH, Markel D. Effect of oral erythromycin therapy in patients with aseptic loosening of joint prostheses. Bone. 2009;44(4):671–7.
Suzuki J, Ogawa M, Hishikari K, Watanabe R, Takayama K, Hirata Y, et al. Novel effects of macrolide antibiotics on cardiovascular diseases. Cardiovasc Ther. 2012;30(6):301–7.
Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: Proposed mechanisms of action. Pharmacol Ther. 2008;117(3):393–405.
Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–91.
Ruygrok PN, Muller DW, Serruys PW. Rapamycin in cardiovascular medicine. Int Med J. 2003;33(3):103–9.
Bosnjakovic A, Mishra MK, Ren W, Kurtoglu YE, Shi T, Fan D, et al. Poly (amidoamine) dendrimer-erythromycin conjugates for drug delivery to macrophages involved in periprosthetic inflammation. Nanomedicine. 2011;7(3):284–94.
Doadrio JC, Sousa EMB, Izquierdo-Barb I, Doadrio AL, Perez-Pariente J, Vallet-Regí M. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J Mater Chem. 2006;16(5):462–6.
Portilla-Arias JA, Camargo B, García-Alvarez M, de Ilarduya AM, Muñoz-Guerra S. Nanoparticles made of microbial poly(γ-glutamate)s for encapsulation and delivery of drugs and proteins. J Biomat Sci. 2009;20(7–8):1065–79.
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, et al. Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit. 2006;19(2):106–80.
Kempe H, Kempe M. Molecularly imprinted polymers. In: Albericio F, Tulla-Puche J, editors. The Power of Functional Resins in Organic Synthesis. Weinheim: Wiley; 2008. p. 15–44.
Alvarez-Lorenzo C, Concheiro A. Molecularly imprinted materials as advanced excipients for drug delivery systems. Biotechnol Annu Rev. 2006;12:225–68.
Kryscio DR, Peppas NA. Mimicking biological delivery through feedback-controlled drug release systems based on molecular imprinting. AIChE J. 2009;55(6):1311–24.
Fernández-González A, Guardia L, Badía-Laíño R, Díaz-García ME. Mimicking molecular receptors for antibiotics – analytical implications. Trends Anal Chem. 2006;25(10):949–57.
Cederfur J, Pei Y, Zihui M, Kempe M. Synthesis and screening of a molecularly imprinted polymer library targeted for penicillin G. J Comb Chem. 2003;5(1):67–72.
Benito-Pena E, Moreno-Bondi MC, Aparicio S, Orellana G, Cederfur J, Kempe M. Molecular engineering of fluorescent penicillins for molecularly imprinted polymer assays. Anal Chem. 2006;78(6):2019–27.
Kempe H, Kempe M. Influence of salt ions on binding to molecularly imprinted polymers. Anal Bioanal Chem. 2010;396(4):1599–606.
Kempe H, Kempe M. QSRR analysis of β-lactam antibiotics on a penicillin G targeted MIP stationary phase. Anal Bioanal Chem. 2010;398(7–8):3087–96.
Levi R, McNiven S, Piletsky SA, Cheong S-H, Yano K, Karube I. Optical detection of chloramphenicol using molecularly imprinted polymers. Anal Chem. 1997;69(11):2017–21.
Mirzaei M, Najafabadi SAH, Abdouss M, Azodi-Deilami S, Asadi E, Hosseini MRM, et al. Preparation and utilization of microporous molecularly imprinted polymer for sustained release of tetracycline. J Appl Poly Sci. 2013;128(3):1557–62.
Shi Y, Lv H, Lu X, Huang Y, Zhang Y, Xue W. Uniform molecularly imprinted poly (methacrylic acid) nanospheres prepared by precipitation polymerization: the control of particle features suitable for sustained release of gatifloxacin. J Mater Chem. 2012;22(9):3889–98.
Siemann M, Andersson LI, Mosbach K. Separation and detection of macrolide antibiotics by HPLC using macrolide-imprinted synthetic polymers as stationary phases. J Antibiot. 1997;50(1):89–95.
Song S, Wu A, Shi X, Li R, Lin Z, Zhang D. Development and application of molecularly imprinted polymers as solid-phase sorbents for erythromycin extraction. Anal Bioanal Chem. 2008;390(8):2141–50.
Geng L, Kou X, Lei J, Su H, Maa G, Su Z. Preparation, characterization and adsorption performance of molecularly imprinted microspheres for erythromycin using suspension polymerization. J Chem Technol Biotechnol. 2012;87(5):635–42.
Kou X, Lei J, Geng L, Deng H, Jiang Q, Zhang G, et al. Synthesis, characterization and adsorption behavior of molecularly imprinted nanospheres for erythromycin using precipitation polymerization. J Nanosci Nanotechnol. 2012;12(9):7388–94.
Zhang Z, Yang X, Zhang H, Zhang M, Luo L, Hu Y, et al. Novel molecularly imprinted polymers based on multi-walled carbon nanotubes with binary functional monomer for the solid-phase extraction of erythromycin from chicken muscle. J Chromatogr B. 2011;879(19):1617–24.
Kempe M, Mosbach K. Receptor binding mimetics: a novel molecularly imprinted polymer. Tetrahedron Lett. 1995;36(20):3563–6.
Kempe M. Antibody mimicking polymers as chiral stationary phases in HPLC. Anal Chem. 1996;68(11):1948–53.
Ye L, Cormack PAG, Mosbach K. Molecularly imprinted monodisperse microspheres for competitive radioassay. Anal Commun. 1999;36(2):35–8.
Connolly ML. Computation of molecular volume. J Am Chem Soc. 1985;107(5):1118–24.
Sibrian-Vazquez M, Spivak DA. Molecular imprinting made easy. J Am Chem Soc. 2004;126(25):7827–33.
Ray RS, Mehrotra S, Shankar U, Suresh Babu G, Joshi PC, Hans RK. Evaluation of UV-induced superoxide radical generation potential of some common antibiotics. Drug Chem Toxicol. 2001;24(2):191–200.
Jedliński Z, Paprotny J. Synthesis and polymerisation of some N-alkylolacryl-amides. III. Polymerization of 2-methacrylamido-2-methyl-propanediol-l,3 and 2-methacrylamido-2-methylpropanol-1. J Polym Sci Part A-1. 1967;5(11):2957–60.
Karlsson JG, Karlsson B, Andersson LI, Nicholls IA. The roles of template complexation and ligand binding conditions on recognition in bupivacaine molecularly imprinted polymers. Analyst. 2004;129(5):456–62.
Zhang Y, Song D, Lanni LM, Shimizu KD. Importance of functional monomer dimerization in the molecular imprinting process. Macromolecules. 2010;43(15):6284–94.
Cacho C, Turiel E, Martin-Esteban A, Pérez-Conde C, Cámara C. Characterisation and quality assessment of binding sites on a propazine-imprinted polymer prepared by precipitation polymerization. J Chromatogr B. 2004;802(2):347–53.
Chen Z, Ye L. Controlling size and uniformity of molecularly imprinted nanoparticles using auxiliary template. J Mol Recognit. 2012;25(6):370–6.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by Greta och Johan Kocks Stiftelser, Stiftelsen Syskonen Svenssons fond för Medicinsk forskning, and Magnus Bergvalls Stiftelse. Dr. Eric Carlemalm and Ms. Birgitta Lindén is acknowledged for help with TEM and BET analysis, respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 726 kb)
Rights and permissions
About this article
Cite this article
Kempe, H., Parareda Pujolràs, A. & Kempe, M. Molecularly Imprinted Polymer Nanocarriers for Sustained Release of Erythromycin. Pharm Res 32, 375–388 (2015). https://doi.org/10.1007/s11095-014-1468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1468-2