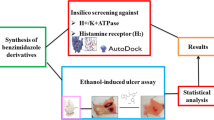
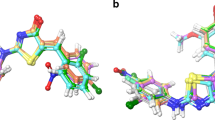
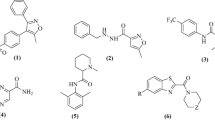
The present work aimed to study the therapeutic significance of various substituted benzimidazole derivatives in the treatment of peptic ulcers. Aspirin induced gastric ulcer model was used for the study of antiulcer activity using acetyl salicylic acid as ulcer inducing agent and albino rats as the screening model. Pharmacological screening protocol for the antiulcer activity of synthesized compounds was based on the aspirin induced pylorus ligation method. The synthesized compounds were estimated by determining biochemical parameters such as pH, ulcer index, free acidity and total acidity. The acute oral toxicity studies were performed as per OECD 423 guidelines. The effect of several synthesized substituted benzimidazole compounds were compared to that of the standard drug omeprazole. It was found that compounds R1, R2 and R8 exhibited potent inhibitory activity. The results of molecular docking showed that compound R8 had a maximum binding affinity for H+/K+ ATPase of –9.3 kcal/mol. The study revealed that compound R8 has a potent activity and can be used as a lead molecule. Physicochemical properties of synthesized drugs were characterized by spectral techniques.







Similar content being viewed by others
References
L. Kuna, J. Jakab, R. Smolic, et al., J. Clin. Med., 8(2), 179 (2019).
A. R. Ramos, J. B. Kirsner, and W. L. Palmer, AMA J. Dis. Children, 99(2), 135 – 148 (1960).
A. O’Connor, T. Furuta, J. P. Gisbert, and C. O’Morain, Helicobacter, 25, e12743 (2020).
A. M. Asali, M. A. Alghamdi, S. A. Fallatah, et al., Int. J. Community Med. Public Health, 5(10), 4617 – 4624 (2018).
J. J. Y. Sung, E. J. Kuipers, and H. B. El-Serag, Aliment. Pharmacol. Ther., 29(9), 938 – 946 (2009).
K. Shah, S. Chhabra, S. K. Shrivastava, P. Mishra, Med. Chem. Res., 22(11), 5077 – 5104 (2013).
M. S. Vasava, M. N. Bhoi, S. K. Rathwa, et al., Mini-Rev. Med. Chem., 20(7), 532 – 565 (2020).
G. E. Rao, P. S. Babu, O. S. Koushik, et al., Int. J. Pharm. Chem. Biol. Sci., 6(2), 227 – 232 (2016).
A. Patil, S. Ganguly, S. Surana, Rasayan J. Chem., 1(3), 447 – 460 (2008).
K. P. Barot, S. Nikolova, I. Ivanov, M. D. Ghate, Mini-Rev. Med. Chem., 13(10), 1421 – 1447 (2013).
G. Yadav and S. Ganguly, Eur. J. Med. Chem., 97, 419 – 443 (2015).
S. Choudhary, M. Arora, H. Verma, et al., Eur. J. Pharmacol., 899, 174027 (2021).
V. A. Vare, T. R. Bagle, and R. C. Hire, Int. J. Basic Clin. Pharmacol., 7(1), 38 (2018).
S. I. Alaqeel, J. Saudi Chem. Soc., 21(2), 229 – 237 (2017).
E. Cereda, M. Turconi, A. Ezhaya, et al., Eur. J. Med. Chem., 22(6), 527 – 537 (1987).
G. Navarette-Vazquez, H. Moreno-Diaz, F. Aguirre-Crespo, et al., Bioorg. Med. Chem. Lett., 16, 4169 – 4173 (2006).
D. I. Shah, M. Sharma, Y. Bansal, et al., Eur. J. Med. Chem., 950 – 969 (2006).
E. Schlede, et al., Arch. Toxicol, 69, 659 – 670 (1994).
A. Patil, S. Ganguly, and S. Surana, J. Chem. Sci., 122(3), 443 – 450 (2010).
H. G. Vogel and W. H. Vogel, Drug Discovery Eval., 52(1998), Biomedicine & Pharmacotherapy, p. 825.
S. Maity, T. Chaudhuri, J. R. Vedasiromoni, and D. K. Ganguly, Indian J. Pharmacol., 35(4), 213 – 219 (2003).
Z. Li, H. Wan, Y. Shi, and P. Ouyang, J. Chem. Inform. Computer Sci., 44(5), 1886 – 1890 (2004).
A. D. Hunter, J. Chem. Educ., 74(8), 905 – 906 (1997).
J. Kirchmair, P. Markt, S. Distinto, et al., J. Med. Chem., 51(22), 7021 – 7040 (2008).
D. S. Goodsell, Cold Spring Harbor Protocols, 5, pdb-prot5200 (2009).
R. Radhamanalan, M. Alagumuthu, and N. Nagaraju, Future Med. Chem., 10(15), 1805 – 1820 (2018).
N. Agarwal, A. Bajpai, V. Srivastava, and S. P. Gupta, Struct. Biol., 2013, 810691 (2013).
S. Sharma, A. Sharma, and U. Gupta, Ann. Antivir. Antiretrovir., 5(1), 028 – 032 (2021).
Acknowledgments
We express our immense debt of gratitude to the Management of C. L. Baid Metha College of Pharmacy for providing us with necessary requirements and facilities at every stage of our project work. Their support and guidance have enabled us to execute the present work successfully.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nalini, C.N., Prabakaran, A. & Jayasree, R. Design, Synthesis, Pharmacological Screening and Molecular Docking Study of New Substituted Benzimidazole Derivatives. Pharm Chem J 57, 51–59 (2023). https://doi.org/10.1007/s11094-023-02850-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02850-z