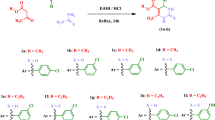
Appearance of drug-unresponsive strains of Leishmania genus and toxic side effects of current chemotherapies necessitate the search for novel antileishmanial agents. Within present contribution, new dihydropyrimidine/thione-5-carboxylates/carboxamide derivatives were synthesized and biologically assessed against Leishmania major promastigotes. A few derivatives exhibited promising antileishmanial activities (IC50s 24.7 – 2223.9 μM). Results of biological assessment revealed compound 4 as the most potent antileishmanial agent. Compounds 1, 3, 4, 8 and 9 exhibited significant activity with regard to Glucantime (control drug). For both 5-carboxamide and 5-carboxylate series, it was found that steric hindrance of 4-(3-substituted phenyl) position was determinant in antileishmanial effect proposing a hydrophobic pocket near meta position of 4-phenyl moiety. Molecular docking approach vs. pteridine reductase 1 as a validated leishmanial target revealed that 5-carboxylate derivatives made H-bond with enzyme cofactor, i.e., nicotinamide adenine dinucleotide phosphate (NDP602). Interaction pattern was relatively similar to previously reported drugs such as trimethoprim. Binding efficiency indices (BEIs) were in accordance with antileishmanial IC50 values.






Similar content being viewed by others
References
WHO (2019): https://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/, visited 20 December 2019.
S. N. Khattab, A. A. Bekhit, A. El-Faham, et al., Chem. Pharm. Bull., 56, 1717 – 1721 (2008).
D. Mukhopadhyay, J. E. Dalton, P. M. Kaye, et al., Trends Parasitol., 30, 65 – 74 (2014).
P. J. Hotez and B. Pecoul, PLoS Neglected Trop. Dis., 4, e718 (2010).
R. Reithinger., J. Dujardin, H. Louzir, et al., Lancet Infect. Dis., 9, 581 – 596 (2007).
A. Ponte-Sucre, F. Gamarro, J. Dujardin, et al., PLoS Neglected Trop. Dis., 12, 1 – 24 (2017).
P. K. Chaudhari, A. Pandey, and V. H. Shah, Orient. J. Chem., 26(4), 1377 – 1383 (2010).
V. Virsodia, D. Manvar, R. R. S. Pissurlenkar, et al., Eur. J. Med. Chem., 43(10), 2103 – 2115 (2008).
N. Foroughifar, S. K. Beromi, H. Pasdar, et al., Iran. J. Pharm. Res., 16(2), 596 – 601 (2017).
T. U. Mayer, T. M. Kapoor, S. J. Haggarty, et al., Science, 268(5441), 971 – 974 (1999).
U. Rashid, R. Sultana, N. Shaheen, et al., Eur. J. Med. Chem., 115, 230 – 244 (2016).
F. Bohlooli, S. Sepehri, N. Razzaghi-Asl, et al., Comput. Biol. Chem., 67, 158 – 173 (2017).
C. Abad-Zapatero and J. M. Metz, Drug Discov. Today, 10(7), 464 – 469 (2005).
C. Abad-Zapatero, Expert Opin. Drug Discov., 2(4), 469 – 488 (2007).
R. Rajasekara and Y.-P. Phoebe Chen, Drug Discov. Today, 20(8), 958 – 968 (2015).
K. F. M Atta, T. M. Ibrahim, O. O. M. Farahat, et al., Future Med. Chem., 9(16), 1913 – 1929 (2017).
L. B. Tulloch, V. P. Martini, J. Iulek, et al., J. Med. Chem., 53(1), 221 – 229 (2010).
S. Safari, R. Ghavimi, N. Razzaghi-Asl, et al., J. Heterocycl. Chem., 57(3), 1023 – 1033 (2020).
A. Niapour, K. Amirshahrokhi, M. Azari Rad, et al., J. Ardabil Univ. Med. Sci., 19(1), 61 – 70 (2019).
M. Heidari-Kharaji, V. Fallah-Omrani, A. Badirzadeh, et al., Parasite Immunol., 41(1), e12605 (2019).
G. M. Morris, R. Huey, W. Lindstrom, et al., J. Comput. Chem., 30, 2785 – 2791, (2009).
M. Sanner, J. Mol. Graphics Mod., 17(1), 57 – 61 (1999).
A. W. Schuettelkopf, L. W. Hardy, S. M. Beverley, et al., J. Mol. Biol., 352, 105 – 116 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi-Ghalehbin, B., Sepehri, S., Nejatkhah, N. et al. Synthesis, Antileishmanial Activity and Molecular Docking Study of New 3,4-Dihydropyrimidinones/Thiones. Pharm Chem J 55, 1050–1056 (2022). https://doi.org/10.1007/s11094-021-02536-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02536-4