Abstract
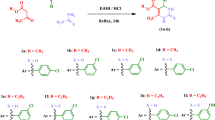
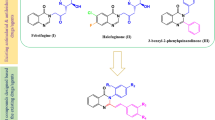
Leishmaniasis includes a range of parasitic diseases caused by numerous types of the protozoan kinetoplastid parasite. Fungal and bacterial pathogens have led to infectious illnesses causing some main public health problem in current years. A series of dihydropyridine and tetrahydropyrimidine derivatives having fluoro, bromo, and nitro substituents at para-phenyl ring on C4 of dihydropyridine and tetrahydropyrimidine rings were synthesized. Then, anti-leishmanial and antimicrobial potencies of compounds were assessed. All compounds were synthesized via Hantzsch and Biginelli reactions. All derivatives were evaluated for their anti-leishmanial and antimicrobial activities. Moreover, docking and molecular dynamics simulation calculations of the compounds in PRT1 binding site were performed to report the results of anti-leishmanial and antimicrobial activities. Compounds 4a and 4b showed the highest anti-amastigote and anti-promastigote activities. Compound 4a revealed the highest antimicrobial activity against E. coli, P. aeruginosa, and C. albicans strains. In addition, compound 4c showed the highest activity against S. aureus. The fluoro, bromo, and nitro substituents in para-position of phenyl group at C4 of dihydropyridine and tetrahydropyrimidine moieties as well as the bulk and length of the chain linking to the ester moieties are essential for anti-leishmanial and anti-microbial activities of these derivatives. Low cytotoxicity was shown by most of derivatives against macrophages. The molecular docking studies were in agreement with in vitro assay. Moreover, hydrogen binds, RMSF, RMSD, and Rg, strongly showed the steady binding of 4a and 4b compounds in PRT1 active site.
Graphical abstract












Similar content being viewed by others
References
Haroon M, de Barros Dias MCH, Santos ACdS, Pereira VRA, Barros Freitas LA, Balbinot RB, Kaplum V, Nakamura CV, Alves LC, Brayner FA, Leite ACL, Akhtar T (2021) The design, synthesis, and in vitro trypanocidal and leishmanicidal activities of 1,3-thiazole and 4-thiazolidinone ester derivatives. RSC Adv 11:2487–2500. https://doi.org/10.1039/D0RA06994A
Steverding D (2017) The history of leishmaniasis. Parasit Vectors 10:82. https://doi.org/10.1186/s13071-017-2028-5
Gontijo VS, Colombo FA, Ferreira Espuri P, Freitas PG, Nunes JB, Alves LB, Veloso MP, Alves RB, Freitas RP, Marques MJ (2021) In vivo evaluation of anti-Leishmania activity of alkyltriazoles and alkylphosphocholines by oral route. Exp Parasitol 226–227:108123. https://doi.org/10.1016/j.exppara.2021.108123
Kapil S, Singh PK, Silakari O (2018) An update on small molecule strategies targeting leishmaniasis. Eur J Med Chem 157:339–367. https://doi.org/10.1016/j.ejmech.2018.08.012
Sheikhmoradi V, Saberi S, Saghaei L, Pestehchian N, Fassihi A (2018) Synthesis and antileishmanial activity of antimony (V) complexes of hydroxypyranone and hydroxypyridinone ligands. Research in pharmaceutical sciences 13:111–120. https://doi.org/10.4103/1735-5362.223793
Gómez-Pérez V, Manzano JI, García-Hernández R, Castanys S, Gamarro F, Campos JM (2015) Design, synthesis and anti-leishmanial activity of novel symmetrical bispyridinium cyclophanes. Eur J Med Chem 89:362–369. https://doi.org/10.1016/j.ejmech.2014.10.040
Nandikolla A, Srinivasarao S, Karan Kumar B, Murugesan S, Aggarwal H, Major LL, Smith TK, Chandra Sekhar KVG (2020) Synthesis, study of antileishmanial and antitrypanosomal activity of imidazo pyridine fused triazole analogues. RSC Adv 10:38328–38343. https://doi.org/10.1039/D0RA07881F
Mendes EP, Goulart CM, Chaves OA, Faiões VDS, Canto-Carvalho MM, Machado GC, Torres-Santos EC, Echevarria A (2019) Evaluation of novel chalcone-thiosemicarbazones derivatives as potential anti-leishmania amazonensis agents and its HSA binding studies. Biomolecules. https://doi.org/10.3390/biom9110643
Zhang L, Feng XZ, Xiao ZQ, Fan GR, Chen SX, Liao SL, Luo H, Wang ZD (2021) Design, synthesis, antibacterial, antifungal and anticancer evaluations of novel β-pinene quaternary ammonium salts. Int J Mol Sci. https://doi.org/10.3390/ijms222011299
Meşeli T, Doğan ŞD, Gündüz MG, Kökbudak Z, Skaro Bogojevic S, Noonan T, Vojnovic S, Wolber G, Nikodinovic-Runic J (2021) Design, synthesis, antibacterial activity evaluation and molecular modeling studies of new sulfonamides containing a sulfathiazole moiety. New J Chem 45:8166–8177. https://doi.org/10.1039/D1NJ00150G
Sapozhnikov SV, Sabirova AE, Shtyrlin NV, Druk AY, Agafonova MN, Chirkova MN, Kazakova RR, Grishaev DY, Nikishova TV, Krylova ES, Nikitina EV, Kayumov AR, Shtyrlin YG (2021) Design, synthesis, antibacterial activity and toxicity of novel quaternary ammonium compounds based on pyridoxine and fatty acids. Eur J Med Chem 211:113100. https://doi.org/10.1016/j.ejmech.2020.113100
Liu HX, Ma DL, Cui G, Zhang Y, Xue FQ (2021) Design, synthesis and antibacterial activities of pleuromutilin derivatives. J Asian Nat Prod Res 23:123–137. https://doi.org/10.1080/10286020.2020.1713764
Bhakhar KA, Sureja DK, Dhameliya TM (2022) Synthetic account of indoles in search of potential anti-mycobacterial agents: a review and future insights. J Mol Struct 1248:131522. https://doi.org/10.1016/j.molstruc.2021.131522
Dhameliya TM, Chudasma SJ, Patel TM, Dave BP (2022) A review on synthetic account of 1,2,4-oxadiazoles as anti-infective agents. Mol Diversity 26:2967–2980. https://doi.org/10.1007/s11030-021-10375-4
Dhameliya TM, Bhakhar KA, Gajjar ND, Patel KA, Devani AA, Hirani RV (2022) Recent advancements and developments in search of anti-tuberculosis agents: a quinquennial update and future directions. J Mol Struct 1248:131473. https://doi.org/10.1016/j.molstruc.2021.131473
Sepehri S, Sanchez HP, Fassihi A (2015) Hantzsch-Type dihydropyridines and Biginelli-type tetra-hydropyrimidines: a review of their chemotherapeutic activities. J Pharm Pharm Sci 18:1–52. https://doi.org/10.18433/j3q01v
Abdolmohammadi S, Mirza B, Vessally E (2019) Immobilized TiO2 nanoparticles on carbon nanotubes: an efficient heterogeneous catalyst for the synthesis of chromeno[b]pyridine derivatives under ultrasonic irradiation. RSC Adv 9:41868–41876. https://doi.org/10.1039/C9RA09031B
Saeedi B, Abdolmohammadi S, Mirjafari Z, Kia-Kojoori R (2020) Nickel(II) chromite nanoparticles promoted efficient synthesis of novel [1]benzopyrano[4,3-b]pyridines in aqueous media. Monatshefte für Chemie—Chem Month 151:773–780. https://doi.org/10.1007/s00706-020-02595-5
Abdolmohammadi S, Dahi-Azar S, Mohammadnejad M, Hosseinian A (2017) A simple and efficient synthesis of 4-arylacridinediones and 6-aryldiindeno[1,2-b:2,1-e]pyridinediones using CuI nanoparticles as catalyst under solvent-free conditions. Comb Chem High Throughput Screen 20:773–780. https://doi.org/10.2174/1386207320666171002123027
Abdolmohammadi S, Hossaini Z, Poor Heravi MR (2022) PANI-Fe3O4@ZnO nanocomposite as magnetically recoverable organometallic nanocatalyst promoted synthesis of new Azo chromene dyes and evaluation of their antioxidant and antimicrobial activities. Mol Diversity 26:1983–1993. https://doi.org/10.1007/s11030-021-10309-0
Chaghari-Farahani F, Abdolmohammadi S, Kia-Kojoori R (2020) A PANI-Fe3O4@ZnO nanocomposite: a magnetically separable and applicable catalyst for the synthesis of chromeno-pyrido[d]pyrimidine derivatives. RSC Adv 10:15614–15621. https://doi.org/10.1039/D0RA01978J
Abdolmohammadi S, Karimpour S (2016) Rapid and mild synthesis of quinazolinones and chromeno[d]pyrimidinones using nanocrystalline copper(I) iodide under solvent-free conditions. Chin Chem Lett 27:114–118. https://doi.org/10.1016/j.cclet.2015.08.014
Rabiei A, Abdolmohammadi S, Shafaei F (2017) A green approach for an efficient preparation of 2, 4-diamino-6-aryl-5-pyrimidinecarbonitriles using a TiO2–SiO2 nanocomposite catalyst under solvent-free conditions. Zeitschrift für Naturforschung B 72:241–247
Fakheri-Vayeghan S, Abdolmohammadi S, Kia Kojoori R (2018) An expedient synthesis of 6-amino-5-[(4-hydroxy-2-oxo-2H-chromen-3-yl)(aryl)methyl]-1,3-dimethyl-2,4,6(1H,3H)-pyrimidinedione derivatives using Fe3O4@TiO2 nanocomposite as an efficient, magnetically separable, and reusable catalyst. Zeitschrift für Naturforschung B. https://doi.org/10.1515/znb-2018-0030
Khalilian S, Abdolmohammadi S, Nematolahi F (2017) An eco-friendly and highly efficient synthesis of pyrimidinones using a TiO2-CNTs nanocomposite catalyst. Lett Org Chem 14:361–367. https://doi.org/10.2174/1570178614666170321113926
Abdolmohammadi S, Afsharpour M (2015) ChemInform abstract: an operationally simple green procedure for the synthesis of dihydropyrimido[4,5-d]pyrimidinetriones using CuI nanoparticles as a highly efficient catalyst. Zeitschrift für Naturforschung B. https://doi.org/10.1515/znb-2014-0207
Kiani M, Abdolmohammadi S, Janitabar-Darzi S (2017) Fast and efficient synthesis of chromeno[d]pyrimidinediones catalysed by a TiO2-SiO2 nanocomposite in aqueous media. J Chem Res 41:337–340. https://doi.org/10.3184/174751917X14949407124706
Gomha SM, Edrees MM, Muhammad ZA, Kheder NA, Abu- Melha S, Saad AM (2022) Synthesis, characterization, and antimicrobial evaluation of some new 1,4-dihydropyridines-1,2,4-triazole hybrid compounds. Polycyclic Aromat Compd 42:173–185. https://doi.org/10.1080/10406638.2020.1720751
Mishra AP, Bajpai A, Rai AK (2019) 1,4-dihydropyridine: a dependable heterocyclic ring with the promising and the most anticipable therapeutic effects. Mini Rev Med Chem 19:1219–1254. https://doi.org/10.2174/1389557519666190425184749
Reddy MU, Reddy MCS, Chakravartula V (2018) Synthesis and anti-bacterial activity of 1,4-dihydropyridine derivatives embedded with chromone and dimedone based hybrids. Asian J Res Chem 11:55–60
Abu-Melha H (2013) Synthesis, antibacterial and antifungal evaluation of novel 1,4-dihydropyridine derivatives. Spectrochim Acta A Mol Biomol Spectrosc 113:115–122. https://doi.org/10.1016/j.saa.2012.12.069
Ahamed A, Arif IA, Mateen M, Surendra Kumar R, Idhayadhulla A (2018) Antimicrobial, anticoagulant, and cytotoxic evaluation of multidrug resistance of new 1,4-dihydropyridine derivatives. Saudi J Biol Sci 25:1227–1235. https://doi.org/10.1016/j.sjbs.2018.03.001
Malani A, Makwana A, Monapara J, Ahmad I, Patel H, Desai N (2021) Synthesis, molecular docking, DFT study, and in vitro antimicrobial activity of some 4-(biphenyl-4-yl)-1,4-dihydropyridine and 4-(biphenyl-4-yl)pyridine derivatives. J Biochem Mol Toxicol 35:e22903. https://doi.org/10.1002/jbt.22903
Foroughifar N, Karimi Beromi S, Pasdar H, Shahi M (2017) Synthesis of some new tetrahydropyrimidine derivatives as possible antibacterial agents. Iran J Pharm Res 16:596–601
Desai NC, Vaghani HV, Patel BY, Karkar TJ (2018) Synthesis and antimicrobial activity of fluorine containing pyrazole-clubbed dihydropyrimidinones. Indian J Pharm Sci 80:242–252
Razzaghi-Asl N, Kamrani-Moghadam M, Farhangi B, Vahabpour R, Zabihollahi R, Sepehri S (2019) Design, synthesis and evaluation of cytotoxic, antimicrobial, and anti-HIV-1 activities of new 1,2,3,4-tetrahydropyrimidine derivatives. Res Pharm Sci 14:155–166. https://doi.org/10.4103/1735-5362.253363
Mirzayi S, Kakanj M, Sepehri S, Alavinejad B, Bakherad Z, Ghazi-Khansari M (2021) Design and synthesis of tetrahydropyrimidinone(thione)-triazole hybrid scaffolds and evaluation of their biological activities. Phosphorus Sulfur Silicon Relat Elem 196:1109–1116. https://doi.org/10.1080/10426507.2021.1986499
Razzaghi-Asl N, Sepehri S, Ebadi A, Karami P, Nejatkhah N, Johari-Ahar M (2020) Insights into the current status of privileged N-heterocycles as antileishmanial agents. Mol Divers 24:525–569. https://doi.org/10.1007/s11030-019-09953-4
Kumar P, Kumar A, Verma SS, Dwivedi N, Singh N, Siddiqi MI, Tripathi RP, Dube A, Singh N (2008) Leishmania donovani pteridine reductase 1: biochemical properties and structure-modeling studies. Exp Parasitol 120:73–79. https://doi.org/10.1016/j.exppara.2008.05.005
Saudi MNS, El-Semary MMA, Elbayaa RY, Jaeda MI, Eissa MM, Amer EI, Baddour NM (2012) Synthesis and biological evaluation of a novel class as antileishmanial agent. Med Chem Res 21:257–267. https://doi.org/10.1007/s00044-010-9532-x
Reimão JQ, Scotti MT, Tempone AG (2010) Anti-leishmanial and anti-trypanosomal activities of 1,4-dihydropyridines: in vitro evaluation and structure-activity relationship study. Bioorg Med Chem 18:8044–8053. https://doi.org/10.1016/j.bmc.2010.09.015
Kaur J, Sundar S, Singh N (2010) Molecular docking, structure-activity relationship and biological evaluation of the anticancer drug monastrol as a pteridine reductase inhibitor in a clinical isolate of Leishmania donovani. J Antimicrob Chemother 65:1742–1748. https://doi.org/10.1093/jac/dkq189
Jeddi B, Saberi S, Menéndez JC, Sepehri S (2021) Synthesis and biological evaluation of tetrahydropyrimidine and dihydropyridine derivatives against leishmania major. Acta Parasitol. https://doi.org/10.1007/s11686-021-00457-6
Rezaei A-R, Saberi S, Sepehri S (2022) Synthesis, antileishmanial activity and molecular docking study of a series of dihydropyridine derivatives. Polycyc Arom Compounds. https://doi.org/10.1080/10406638.2022.2092877
Mohammadi-Ghalehbin B, Sepehri S, Nejatkhah N, Safari S, Hosseinali Z, Sabour S, Razzaghi-Asl N (2022) Synthesis, antileishmanial activity and molecular docking study of new 3,4-dihydropyrimidinones/thiones. Pharm Chem J 55:1050–1056. https://doi.org/10.1007/s11094-021-02536-4
Bansod PS, Jadhav SB (2022) Design, molecular docking studies and ADMET prediction of chalcones of indole-benzenesulfonyl derivatives as thioredoxin inhibitor for anticancer activity. J Comput Biophys Chem. https://doi.org/10.1142/s2737416522500144
Chandrasekaran S, Veronica J, Gundampati RK, Sundar S, Maurya R (2016) Exploring the inhibitory activity of Withaferin-A against Pteridine reductase-1 of L. donovani. J Enzyme Inhib Med Chem 31:1029–1037. https://doi.org/10.3109/14756366.2015.1088841
Shraddha P, Rakesh S, Devender P (2020) New benzimidazole derivatives as inhibitors of Pteridine reductase 1: design, molecular docking study and ADMET prediction. J Appl Pharma Sci. https://doi.org/10.7324/JAPS.2020.10904
Di Pisa F, Landi G, Dello Iacono L, Pozzi C, Borsari C, Ferrari S, Santucci M, Santarem N, Cordeiro-da-Silva A, Moraes CB, Alcantara LM, Fontana V, Freitas-Junior LH, Gul S, Kuzikov M, Behrens B, Pöhner I, Wade RC, Costi MP, Mangani S (2017) Chroman-4-one derivatives targeting pteridine reductase 1 and showing anti-parasitic activity. Molecules. https://doi.org/10.3390/molecules22030426
Ganesan A, Coote ML, Barakat K (2017) Molecular dynamics-driven drug discovery: leaping forward with confidence. Drug Discov Today 22:249–269. https://doi.org/10.1016/j.drudis.2016.11.001
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45:2615–2623. https://doi.org/10.1021/jm020017n
Asham H, Bohlooli S, Dostkamel D, Rezvanpoor S, Sepehri S (2021) Design, synthesis, and biological screening for cytotoxic activity of monastrol analogues. Polycyc Arom Compounds. https://doi.org/10.1080/10406638.2021.1913424
Keivanloo A, Fakharian M, Sepehri S (2020) 1,2,3-Triazoles based 3-substituted 2-thioquinoxalines: synthesis, anti-bacterial activities, and molecular docking studies. J Mol Struct 1202:127262. https://doi.org/10.1016/j.molstruc.2019.127262
Sepehri S, Hashemidanesh N, Mahnam K, Asham H (2021) Qualitative and quantitative analysis of anti-viral compounds against SARS-CoV-2 protease enzyme by molecular dynamics simulation and MM/PBSA method. Pharm Sci 27:S122–S134. https://doi.org/10.34172/ps.2021.4
Bhakhar KA, Gajjar ND, Bodiwala KB, Sureja DK, Dhameliya TM (2021) Identification of anti-mycobacterial agents against mmpL3: virtual screening, ADMET analysis and MD simulations. J Mol Struct 1244:130941. https://doi.org/10.1016/j.molstruc.2021.130941
Funding
This research was supported by Ardabil University of Medical Sciences. Funding Number is IR.ARUMS.REC.1398.092
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalilzadeh, M., Saberi, S., Noori, G. et al. Synthesis, biological assessment, and computational investigations of nifedipine and monastrol analogues as anti-leishmanial major and anti-microbial agents. Mol Divers 27, 2555–2575 (2023). https://doi.org/10.1007/s11030-022-10569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10569-4