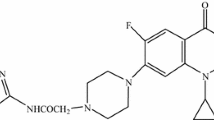
The increasing global health problem of bacterial resistance to the major classes of antibiotics is driving scientists to search for newer antimicrobial agents. The present work was designed to synthesize and evaluate the antimicrobial activities of a new series of sparfloxacin derivatives. Aseries of 22 new sparfloxacin derivatives (1 – 22) were synthesized followed by their spectral characterization and antimicrobial evaluation using serial dilution method. QSAR studies were performed to relate their antimicrobial activity and structure. The results of antimicrobial activity testing against all the three selected bacterial strains (Bacillus subtilis, Staphylococcus aureus, and Escherichia coli) indicated that compounds 21 and 22 exhibited maximum antibacterial potential among all the synthesized sparfloxacin derivatives. Compounds 6, 17, and 22 exhibited maximum antifungal potential against two fungal strains (Candida albicans and Aspergillus niger). The results of QSAR studies revealed the fact that topological parameters, particularly the valence third-order molecular connectivity index, are the major factor in influencing the antibacterial potential of the synthesized molecules. These new derivatives can offer new avenues in the design of better antimicrobial molecules active against drug-resistant bacterial and fungal strains.


Similar content being viewed by others
References
H. Bayrak, A. Demirbas, N. Demirbas, and S. A. Karaoglu, Eur. J. Med. Chem., 44, 4362 (2009).
V. Judge, B. Narasimhan, M. Ahuja, et al., J. Balzarini, Med. Chem. Res., 21, 1451 (2012).
U. K. Komarnicka, R. Starosta, K. Guz-Regner, et al., J. Mol. Str., 1096, 55 (2015).
P. C. Sharma, A. Jain, and S. Jain, Acta Pol. Pharm. Drug Res., 66, 587 (2009).
P. C. Sharma, A. Jain, S. Jain, et al., J. Enzyme Inhib. Med. Chem., 25, 557 (2010).
K. C. Fang, Y. L. Chen, J. Y. Sheu, et al., J. Med. Chem., 43, 3809 (2000).
N. Minovski, M. Vracko, and T. Solmajer, Mol. Divers., 15, 417 (2011).
S. Bazile, N. Moreau, D. Bouzard, and M. Essiz, Antimicrob. Agents Chemother., 36, 2622 (1992).
G. E. D. A. A. Abuo-Rahma, H. A. Sarhan, and G. F. M. Gad, Bioorg. Med. Chem., 17, 3879 (2009).
N. Sultana, A. Naz, B. Khan, et al., Med. Chem. Res., 19, 1210 (2010).
S. N. Pandeya, D. Sriram, G. Nath, and E. D. Clercq, Eur. J. Med. Chem., 35, 249 (2000).
D. Sriram, P. Yogeeswari, J. S. Basha, et al., Bioorg. Med. Chem., 13, 5574 (2005).
D. Sriram, A. Aubry, P. Yogeeswari, and L. M. Fisher, Bioorg. Med. Chem., 16, 2982 (2006).
J. G. Cappucino abd N. Sherman, Microbiology: A Laboratory Manual, 7th edn., Pearson Education India, Delhi (2005), p. 129.
Pharmacopoeia of India: Controller of Publications, Vol. I, Ministry of Health Department, Govt. of India, New Delhi (2007), p. 41.
K. J. Schaper, Quant. Struct. Act. Relat., 18, 354 (1999).
D. Sriram, P. Yogeeswari, and S. P. Reddy, Bioorg. Med. Chem. Lett., 16, 2113 (2006).
M. Dinakaran, P. Senthilkumar, P. Yogeeswari, et al., Bioorg. Med. Chem., 18, 1229 (2008).
K. Manna and Y. K. Agrawal, Eur. J. Med. Chem., 45, 3831 (2010).
C. Hansch and T. Fujita, J. Am. Chem. Soc., 86, 1616 (1964).
R. Narang, B. Narasimhan, S. Sharma, et al., Med. Chem., 9, 249 (2013).
A. Golbraikh and A. Tropsha, J. Mol. Graphics Modell., 20, 269 (2002).
Acknowledgments
The authors are thankful to Management, JCD Vidyapeeth, Sirsa (Haryana) for their support and encouragement for this research work.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Grewal, A.S., Singh, V. et al. Synthesis, Antimicrobial Activity and QSAR Studies of Some New Sparfloxacin Derivatives. Pharm Chem J 52, 444–454 (2018). https://doi.org/10.1007/s11094-018-1837-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1837-y