Abstract
A hollow cathode discharge with a CuNi (Cu50Ni50) cathode was operated in an Ar/N\(_2\)/O\(_2\) gas mixture. The energy distribution of plasma ions is investigated with the help of energy-resolved mass spectrometry. Formation of singly ionised Ar\(^+\) and of Cu\(^+\) and Ni\(^+\) ions is observed in pure argon. With the addition of N\(_2\) or O\(_2\) gas the additional formation of molecular N\(_2^ +\) or O\(_2^+\) ions is observed. The intensity of these ions is reduced in the Ar+N\(_2\)+O\(_2\) gas mixture and molecular NO\(^+\) ions become the most abundant ionic species. The formation of neutral NO molecules is confirmed by optical emission spectroscopy. Gas samples collected at the exhaust of the vacuum chamber confirm the formation of NO and, additionally, of NO\(_2\) molecules.
Graphic Abstract
Sample taken from the exhaust of the plasma chamber with a liquid-nitrogen-cooled glass container showing solid nitrogen oxide (blue).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hollow cathode (HC) discharge was discovered by Bartels and Paschen more than 100 years ago [1]. The discharge is sustained by electrons undergoing a pendulum motion inside the HC [2,3,4,5,6,7]. Hollow cathode discharges are employed in many applications, e.g., in atomic spectroscopy [1, 8,9,10], mass spectrometry [11], and laser technology [12], as UV generators [13, 14] and propulsion thrusters [15, 16], for the generation of neutral and ion beams [17, 18], surface processing [19], thin film deposition [20,21,22,23,24,25,26,27], and in plasma chemical investigations [28,29,30,31,32,33,34].
Formation of nitric oxide (NO) has attracted much attention in previous years. Nitric oxide (NO) is a colourless toxic gas which forms by oxidation of nitrogen. It has rather few industrial applications. NO plays a role in combustion and is a flue gas generated, e.g., by automotive petrol engines and fossil fuel power plants [35, 36]. In general, production of nitric oxide during combustion is easier to achieve compared to its removal from the exhaust gas [37,38,39]. Nitric oxide is naturally produced by lightning in thunderstorms and in the upper mesosphere and lower thermosphere (65–140 km) by precipitating energetic electrons and protons during space weather events [40].
Nitric oxide is a small molecule which has wide range of physiological functions in living organisms. In plants, nitric oxide regulates plant metabolism and is involved in many physiological processes, in particular, germination, flowering, or leaf senescence [41, 42]. In mammals, NO is produced in cavernosal nerves and endothelial cells by nitric oxide synthase (NOS) enzymes. NO shows beneficial effect on the healing of skin wounds [43], regulates several physiological procesesses, e.g., blood pressure and hormone release, and plays a key role as a vasodilator of blood vessels and in the physiology of penile erection [44,45,46]. In 1998, the Nobel Prize in Physiology or Medicine 1998 was awarded jointly to Robert F. Furchgott, Louis J. Ignarro and Ferid Murad "for their discoveries concerning nitric oxide as a signalling molecule in the cardiovascular system" [47, 48].
NO formation via plasma chemical reactions has been investigated with different discharges, e.g., glow [49], streamer [50], arc [51], dielectric barrier [52], and microwave discharges [53,54,55,56]. In the present communication we utilize a pulsed hollow cathode (PHC) discharge for the investigation of plasma chemical reactions in a dilute Ar+N\(_2\)+O\(_2\) gas environment. We expect that different nitrogen oxides, in particular, nitric oxide (NO) and nitrogen dioxide (NO\(_2\)) will be generated. The use of a hollow cathode has several advantages compared to other plasma devices. Firstly, the plasma is confined by the walls of the hollow cathode which should give rise to large radical densities without the need of gas heating. Secondly, the properly chosen wall material could serve as a catalyst to further promote the desired chemical reactions [57]. The PHC is operated with a pulse length of 100 \(\mu\)s and a repetition frequency of 5 kHz (duty cycle 50 %). Plasma characterisation and, in particular, plasma chemical processes are studied with the help of energy-resolved mass spectrometry, by optical emission spectroscopy, and by Fourier transform infrared (FTIR) spectroscopy of the exhaust gas.
Experiment
The experimental set-up has been described before [26, 58, 59]. Very briefly, the set-up consists of a cylindrical hollow cathode (HC) inside a vacuum chamber (Fig. 1). The HC is made from copper nickel (Cu50Ni50) alloy. Argon, oxygen, and nitrogen gas are introduced into the vacuum chamber through the hollow cathode. The Ar gas flow rate is typically set to 200 sccm; nitrogen and oxygen gas flow rates are varied up to 100 sccm. The gas pressure directly at the nozzle outlet and inside the HC is significantly larger and in the range of a few 100 Pa. Under these conditions, the expected gas flow speed is sonic at the nozzle’s outlet and becomes supersonic inside the vacuum chamber [22, 26]. The large flow velocity could cause a periodic structure with a barrel shock created by a system of shock waves in the plasma inside the reactor chamber, as observed by Tichy et al. [6].
a Experimental set-up with vacuum chamber showing arrangement of hollow cathode (HC), anode, Langmuir probe, EQP analyser, optical window, and optical spectrograph (schematic). b Electronic arrangement showing cathode power supply, electronic switch, 100 \(\Omega\) resistor, pulse generator (5 kHz), and anode power supply
Temporal evolution of a discharge voltage and b discharge current of a PHC discharge with a Cu50Ni50 nozzle. Pulse length 100 \(\mu\)s, repetition frequency 5 kHz, anode voltage +30 V, mean discharge current 0.25 A. Argon gas flow rate 200 sccm, nitrogen gas flow rate 10 sccm, oxygen gas flow rate 10 sccm, Ar gas pressure \(p=2.1\) Pa
The hollow cathode is connected via a 100 \(\Omega\) resistor to a home-built power switch which is powered by a direct current (DC) power supply. The repetition frequency f and the pulse length T of the pulsed power switch are set to \(f=5\) kHz and \(T_{\textrm{on}}=100\,\mu\)s. The pulsed hollow cathode (PHC) discharge is operated with a mean discharge current of 0.25 A. During operation, the HC heats up to a temperature around 1,000 K as indicated by a red color. An optional ring anode with an inner diameter of 63 mm is mounted at a distance of 100 mm from the hollow cathode [58,59,60].
Energy-resolved mass spectrometry is performed with a commercial Hiden EQP 1000 mass/energy analyzer [61, 62]. The instrument is mounted opposite to the hollow cathode and the anode [58,59,60].
Optical emission spectroscopy (OES) is carried out with a Shamrock SR500D spectrometer equipped with an iCCD detector. The spectrometer is equipped with three gratings having 600 lines/mm, 1800 lines/mm, and 2400 lines/mm and blaze wavelengths of 500 nm, 500 nm, and 300 nm, respectively. An optical fibre connected to the entrance slit of the spectrometer is installed outside the vacuum chamber. It views the open end of the HC at an angle of 38\(^{\textrm{o}}\) with respect to the hollow cathode’s axis through a quartz window with a cut-off wavelength below 200 nm.
Ex-situ Fourier-transform infrared (FTIR) spectoscopy is employed to analyse the exhaust gas from the plasma chamber. Exhaust gas constituents are trapped in a liquid nitrogen-cooled glass container which is inserted into the exhaust line of the pumping system between the turbomolecular pump and the roughing pump. After removal from the exhaust line, the cold gas container is connected to a glass cell with ZnSe windows and optical path of 28 cm. The cell is inserted into the FTIR spectrometer where the sample warms up to room temperature. A high-resolution infrared Fourier-transform spectrometer (Bruker IFS 125HR, Bruker GmBH, Karlsruhe, Germany) is operated in the mid-infrared region between 600 cm\(^{-1}\) and 6000 cm\(^{-1}\) using a KBr beamsplitter and HgCdTe liquid-N\(_2\)-cooled detector. The measured spectra are averaged over 10 scans [63, 64]. The spectra are apodized with a Boxcar apodization function and analysed using the spectr library to obtain partial pressures of the detected components [65].
Results
Discharge Characteristics
The discharge voltage required to maintain a mean discharge current of 0.25 A depends on the employed gas mixture (Fig. 2). Pure Ar requires the smallest discharge voltage of about −425 V. Larger discharge voltages of about −470 V and −490 V are needed for the Ar+O\(_2\) and Ar+O\(_2\)+N\(_2\), respectively. The largest discharge voltage of –540 V is required to sustain the Ar+N\(_2\) gas mixture.
Except for some overshoots that occur right at the beginning of the pulse, all discharge currents increase monotonically with time up to the end of the pulse. Some differences between the gas mixtures are noted. In the case of pure Ar, the discharge current increases smoothly, while in the case of the gas mixtures Ar+O\(_2\), Ar+N\(_2\) and Ar+O\(_2\)+N\(_2\), a kink occurs after about 45 \(\mu\)s, followed by a gradual leveling off. No explanation for this behaviour has been found yet.
Ion mass spectra of a PHC discharge with a Cu50Ni50 nozzle operated in a Ar, b Ar+N\(_2\), c Ar+O\(_2\), and d Ar+N\(_2\)+O\(_2\) gas mixtures. Pulse length 100 \(\mu\)s, repetition frequency 5 kHz, anode voltage +30 V, mean discharge current 0.25 A. Argon gas flow rate 200 sccm, nitrogen gas flow rate 10 sccm, oxygen gas flow rate 10 sccm, Ar gas pressure \(p=2.1\) Pa. Detected ion energy 27 eV. Vertical dashed lines indicate the \(m/z=30\) position
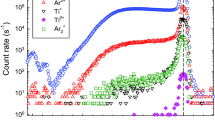
Ion energy distribution of N\(_2^+\) (\(\circ )\), NO\(^+\) (\(\triangle\)), O\(_2^+\) (\(\diamond )\), Ar\(^+\) (\(\triangledown\)), and NO\(_2^+\) ions for a PHC discharge with a positively biased anode (+30 V) in an Ar+O\(_2\)+N\(_2\) gas mixture. Ar gas flow rate 200 sccm, O\(_2\) gas flow rate 10 sccm, N\(_2\) gas flow rate 10 sccm, Ar gas pressure 2.2 Pa, discharge current 0.25 A
Ion Mass and Ion Energy Distribution
Typical ion mass spectra of a PHC discharge operated in different gas mixtures are displayed in Fig. 3. The mass spectrum from pure Ar is dominated by singly-charged Ar\(^+\) (\(m/z=40\)), Ar\(^{2+}\) (\(m/z=20\)), Ni\(^+\) (\(m/z=58\) and 60) and Cu\(^+\) (\(m/z=63\) and 65) ions, where m and z are ion mass and charge number, respectively. The spectrum also contains a mass peak at \(m/z =18\) which is attributed to H\(_2\)O\(^+\) impurity ions. The mass spectrum from the Ar+N\(_2\) or Ar+O\(_2\) gas mixtures additionally display strong mass peaks of N\(_2^+\) (\(m/z=28\)) or O\(_2^+\) (\(m/z=32\)), respectively, ions. Also present are peaks at \(m/z=14\) and \(m/z=16\) of N\(^+\) and O\(^+\) ions, respectively. The most dominant mass peak of the Ar+N\(_2\)+O\(_2\) gas mixture at \(m/z=30\) is attributed to the formation of NO\(^+\) ions. This mass peak is virtually absent in the other mass spectra. In addition, formation of NO\(_2^+\) (\(m/z=46\)) is noted. Compared to the ion mass spectra from the Ar+N\(_2\) or Ar+O\(_2\) gas mixtures, the intensities of N\(_2^+\) or O\(_2^+\) ions, respectively, are considerably reduced. This is a clear indication that NO\(^+\) ions (and NO molecules) are generated through plasma chemical reactions of nitrogen and oxygen species.
Figure 4 compares ion energy distributions of N\(_2^+\) (\(m/z=14\)), O\(_2^+\) (\(m/z=32\)), and Ar\(^+\) (\(m/z=40\)) ions obtained with the Ar+N\(_2\)+O\(_2\) gas mixture. Also shown are results for NO\(^+\) (\(m/z=30\)) and NO\(_2^+\) (\(m/z=46\)) ions which originate from plasma chemical reactions. A positively biased anode (+30 V) to stabilize the discharge and the enhance the kinetic energy of plasma ions is employed during these measurements. The intensity maximum (peak) of the ion energy distributions is shifted to a kinetic energy close to \(E=e_0 V_p\), where \(e_0\) is the elementary charge and \(V_p\) is the plasma potential which is controlled by the anode voltage [58, 59].
The measured energy distributions show a complicated structure with typically two maxima. The energetic position of the high-energy maximum corresponds to the plasma potential close to the anode. The measured kinetic energy where the high-energy maximum occurs is somewhat smaller than expected from the applied anode voltage. It is a typical phenomenon for an oxygen-containing disharge and, e.g., not observed for a HC discharge in pure argon. The Ar\(^+\) ions display a pronounced low-energy maximum which is caused by resonant charge exchange reactions and thus does not represent the plasma potential in the vicinity of the anode [58].
Optical emission spectrum of a PHC discharge with a Cu50Ni50 nozzle operated in a Ar, b Ar+N\(_2\), and c Ar+N\(_2\)+O\(_2\) gas mixtures. Wavelengths of (\(\nu '=0,\nu ''=0-4\)), (\(\nu '=1,\nu ''=1-5\)), and (\(\nu '=2,\nu ''=2-7\)) transitions of the NO \(\gamma\)-bands are indicated. Optical grating with 2,400 lines/mm and a blaze wavelength of 300 nm. Pulse length 100 \(\mu\)s, repetition frequency 5 kHz, mean discharge current 0.25 A. Argon gas flow rate 200 sccm, nitrogen gas flow rate 10 sccm, oxygen gas flow rate 10 sccm, Ar gas pressure \(p=2.1\) Pa
Energy-integrated ion intensities are displayed in Figs. 5 and 6 as function of N\(_2\) and O\(_2\), respectively, gas flow rates. The intensity of N\(_2^+\) ions shows an approximately linear increase with increasing N\(_2\) gas flow rate (Fig. 5). The intensity of NO\(^+\) ions monotonically increases with increasing N\(_2\) gas flow rate. The intensity of NO\(_2^+\) ions also increases with increasing N\(_2\) gas flow rate, however, reaches a maximum at a gas flow rate of \(\approx 2\) sccm and continues with a pronounced decline. The O\(_2^+\) intensity shows a monotonic decrease while the Ar\(^+\) intensity is little influenced by the N\(_2\) gas flow rate. It indicates that O\(_2\) is consumed by the formation of NO\(^+\) ions.
As a function of the O\(_2\) gas flow rate (Fig. 6), the NO\(^+\) ion intensity shows an initial increase, saturates at a gas flow rate of \(\approx 15\) sccm, and remains essentially constant beyond this point. N\(_2^+\) and Ar\(^+\) ion intensities gradually decrease with increasing O\(_2\) flow rate. It may reflect the changing plasma and surface conditions due to the admittance of O\(_2\) through the cathode.
Optical Emission Spectra
Optical emission spectra in the wavelength range 220–280 nm are displayed in Fig. 7. It is the region where we expect the \(\gamma\)-bands (A \(^2\Sigma\) \(\rightarrow\) X \(^2\Pi _r\)) of nitric oxide [66,67,68,69]. The pure Ar spectrum shows several prominent neutral copper (Cu I) lines at 222.57 nm, 244.16 nm, 249.21 nm, 261.84 nm, and 276.64 nm [70, 71]. Neutral nickel (Ni I) lines are observed at 230.08 nm, 231.23/231.37/231.40 nm, 232.00/232.14 nm, and 234.55 nm. Ionized copper (Cu II) lines are present at 224.70 nm, 227.63 nm, and 236.99 nm. Ionized nickel (Ni II) lines are observed at 228.77 nm, 237.54 nm, 239.45 nm, 241.61 nm, and 243.79 nm.
Similar conclusions can be drawn from the emission spectrum of the Ar+N\(_2\) gas mixture. By contrast, the emission spectrum of the Ar+N\(_2\)+O\(_2\) gas mixture is largely different. The spectrum displays several broad emission bands which are attributed to the NO \(\gamma\)-bands. The band heads of the relevant (\(\nu '\), \(\nu ''\)) transitions [72] are indicated in the lower part of Fig. 7c. The present result is a clear indication for the formation of NO molecules in a hollow cathode discharge. Optical emission spectra for different N\(_2\) gas flow rates measured in the spectral range 249–276 nm are displayed in Fig. 8. A linear increase of the integrated OES intensity with N\(_2\) gas flow rate is observed.
Optical emission spectrum of a PHC discharge with a Cu50Ni50 nozzle operated in an Ar+N\(_2\)+O\(_2\) gas mixture for different nitrogen gas flow rates. Wavelengths of (\(\nu '=0,\nu ''=0-4\)), (\(\nu '=1,\nu ''=1-5\)), and (\(\nu '=2,\nu ''=2-7\)) transitions of the NO \(\gamma\)-bands are indicated. Optical grating with 600 lines/mm and a blaze wavelength of 500 nm. Pulse length 100 \(\mu\)s, repetition frequency 5 kHz, mean discharge current 0.25 A. Argon gas flow rate 200 sccm, oxygen gas flow rate 50 sccm, Ar gas pressure \(p=2.1\) Pa
Samples taken from the exhaust line are examined by FTIR spectroscopy. Gas flow rates of 200 sccm, 10 sccm, and 10 sccm for Ar, N\(_2\), and O\(_2\), respectively, are employed and the discharge current is set to 0.25 A. Nitric oxide has a melting point of 109 K [73] and, hence, is, readily captured by the liquid-nitrogen-cooled surface. The analysed spectrum is shown in Fig. 9. The total volume of the sampled gas (at standard conditions) after 60 min of discharge operation is about 80 cm\(^3\). The sample contains a large amount of NO, NO\(_2\), and a small fraction of N\(_2\)O. The relative composition of the observed nitrogen oxides are shown in table 1. In addition, a significant amount of water (H\(_2\)O, about 20 cm\(^3\)) and a rather small amount of CO\(_2\) are present whose origins are not known.
FTIR of a PHC discharge with a Cu50Ni50 nozzle. a Full range and b expanded range showing the NO\(_2\) band at 2907 cm\(^{-1}\). Sampling time 60 min. Pulse length 100 \(\mu\)s, repetition frequency 5 kHz, anode voltage +30 V, mean discharge current 0.25 A. Argon gas flow rate 200 sccm, nitrogen gas flow rate 10 sccm, oxygen gas flow rate 10 sccm, gas pressure \(p=2.3\) Pa
Discussion
The formation of NO molecules in a N\(_2\) + O\(_2\) gas mixture requires the dissociation of N\(_2\) and O\(_2\) molecules into N and O radicals, either by thermal processes at suffiently high gas temperatures, or, as is the case here, in a non-thermal plasma by electron impact [74],
where X stands for N or O. Once formed, N and O radicals can interact with O\(_2\) and N\(_2\), respectively, molecules via the so-called Zeldovich mechanism [74,75,76,77,78,79,80,81]
and
The Zeldovich mechanism is one of the most important reactions for nitric oxide (NO) formation. At the gas temperatures of interest here, reaction 3 is more important since due to the about one order of magnitude smaller activation energy its rate coefficient is several orders of magnitude larger compared to reaction 2.
In the following we anticipate that the majority of the detected ions has formed via neutral-neutral reactions and subsequently are ionised by electron impact in the plasma. The observed ion intensities, hence, reflect the densities of the corresponding neutral species. The ion intensities shown in Fig. 5 indicate that NO formation is a function of N\(_2\) gas density and as such increases with the N\(_2\) gas flow rate. Simultaneously, the density of O\(_2\) decreases as corroborated by the decreasing O\(_2^+\) intensity. Evidently, much of the supplied oxygen is consumed.
Although only a fraction of N\(_2\) has been consumed so far, the NO\(^+\) ion intensity already reaches its saturation level at an O\(_2\) gas flow rate of about 15 sccm (Fig. 6). The saturation coincides with a pronounced increases of the NO\(_2^+\) density which is readily understood by the larger oxygen supply. It can be explained by a further oxidation of NO to NO\(_2\), e.g., via [82, 83]
where M = N\(_2\), O\(_2\), or Ar. A different behaviour is noted as a function of N\(_2\) gas flow rate where the NO\(_2^+\) intensity reaches a maximum at about 2 sccm and then declines (Fig. 5). With increasing N\(_2\) density the number of N radicals increases and back-reactions like
may play a significant role [82, 84].
Conclusions
Plasma-chemical reactions in a pulsed hollow cathode discharge were investigated. A gas mixture of Ar+N\(_2\)+O\(_2\) was used. The formation of nitrogen oxides, especially NO and NO\(_2\), was observed. NO is the dominant molecular species formed in the discharge. Other nitrogen oxides such as N\(_2\)O and NO\(_2\) are produced in smaller amounts. The proportion of other nitrogen oxides depends on the N\(_2\) and O\(_2\) gas flow rates and the N\(_2\) to O\(_2\) ratio. The total amount of nitrogen oxides, mostly NO and NO\(_2\), captured in the exhaust line with a liquid nitrogen-cooled glass container is about 60 cm\(^3\). The role played by the employed CuNi nozzle is not investigated and with respect to eventual catalytic properties remains as a future issue.
Data Availability Statement
Data will be made available upon reasonable request.
References
Paschen F (1916) Bohrs Heliumlinien. Ann Phys IV 50:901. https://doi.org/10.1002/andp.19163551603
Hagelaar GJM, Mihailova DB, van Dijk J (2010) Analytical model of a longitudinal hollow cathode discharge. J Phys D Appl Phys 43:465204. https://doi.org/10.1088/0022-3727/43/46/465204
Kolobov VI, Tsendin LD (1995) Analytic model of the hollow cathode effect. Plasma Sources Sci Technol 4:551. https://doi.org/10.1088/0963-0252/4/4/006
Boeuf JP, Pitchford LC (1995) Field reversal in the negative glow of a DC glow discharge. J Phys D Appl Phys 28:2083. https://doi.org/10.1088/0022-3727/28/10/013
Timmermans CJ, Lunk A, Schram DC (1981) The rotation of ions and neutrals in a cylindrical magnetized hollow cathode argon arc. Beitr Plasmaphys 21:117. https://doi.org/10.1002/ctpp.19810210206
Tichy M, Hubicka Z, Sicha M, Cada M, Olejnicek J, Churpita O, Jastrabik L, Virostko P, Adamek P, Kudrna P, Leshkov S, Chichina M, Kment S (2009) Langmuir probe diagnostics of a plasma jet system. Plasma Sources Sci Technol 18:014009. https://doi.org/10.1088/0963-0252/18/1/014009
Goebel DM, Becatti G, Mikellides IG, Ortega AL (2021) Plasma hollow cathodes. J Appl Phys 130:050902. https://doi.org/10.1063/5.0051228
Duquette DW, Lawler JE (1982) Radiative lifetimes in Nb I. Phys Rev A 26:330. https://doi.org/10.1103/PhysRevA.26.330
Chomski M, Furmann B, Suski M, Głowacki P, Stefanska D, Mieloch S (2023) Determination of the energies of new electronic levels of the holmium atom and investigation of their hyperfine structure. J Quant Spectrosc Radiat Transfer 297:108480. https://doi.org/10.1016/j.jqsrt.2022.108480
Zhou C, Shang X (2023) Experimental branching fractions, transition probabilities, and oscillator strengths in Ni I. J Quant Spectrosc Radiat Transfer 298:108484. https://doi.org/10.1016/j.jqsrt.2023.108484
Ganeev AA, Kuz’menkov MA, Lyubimtsev VA, Potapov SV, Drobyshev AI, Potemin SS, Voronov MV (2007) Pulsed Discharge in a Hollow Cathode with the Detection of Ions in a Time-of-Flight Mass Spectrometer: Analytical Capabilities in the Analysis of Solid Samples. J Anal Chem 62:444. https://doi.org/10.1134/S1061934807050097
Remigy A, Aubert X, Prasanna S, Gazeli K, Invernizzi L, Lombardi G, Lazzaroni C (2022) Absolute N-atom density measurement in an Ar/N\(_2\) micro-hollow cathode discharge jet by means of ns-two-photon absorption laser-induced fluorescence. Phys Plasmas 29:113508. https://doi.org/10.1063/5.0110318
Takashima S, Arai S, Hori M, Goto T, Kono A, Ito M, Yoneda K (2001) Development of vacuum ultraviolet absorption spectroscopy technique employing nitrogen molecule microdischarge hollow cathode lamp for absolute density measurements of nitrogen atoms in process plasmas. J Vac Sci Technol, A 19:599. https://doi.org/10.1116/1.1340655
Choi P, Favre M (1998) Fast pulsed hollow cathode capillary discharge device. Rev Sci Instr 69:3118. https://doi.org/10.1063/1.1149069
Takahashi T, Kinefuchi K (2023) Low power arcjet thruster using LaB6 hollow cathode. Acta Astronaut 206:89. https://doi.org/10.1016/j.actaastro.2023.02.015
Tian F, Miao L, Xia Q, Liang F, Wang N, Hou X (2023) Analysis of the effect of keeper working conditions on hollow cathode performance. Acta Astronaut 211:130. https://doi.org/10.1016/j.actaastro.2023.05.044
Wang F-F, Meng T-H, Da-Ren Yu, Ning Z-X, Zhu X-M (2022) Presence of energetic ions in hollow cathode discharge with low frequency oscillations. J Phys D Appl Phys 55:455202. https://doi.org/10.1088/1361-6463/ac90ce
Lobanov NR, Tunningley T, Linardakis P, Vvedenskaia EG (2023) Insights into the effect of hollow cathode with external injection of fast ions in a sputter-type negative ion source, Nuclear instruments and methods in physics research section a: accelerators. Spectrom Detect Assoc Equip 1048:167933. https://doi.org/10.1016/j.nima.2022.167933
Barankova H, Bardos L (2006) Hollow cathode and hybrid plasma processing. Vacuum 80:688. https://doi.org/10.1016/j.vacuum.2005.11.047
Hubicka Z, Cada M, Adamek P, Virostko P, Olejnicek J, Deyneka A, Jastrabik L, Jurek K, Suchaneck G, Guenther M, Gerlach G, Bohac P (2005) Investigation of the RF pulse modulated plasma jet system during the deposition of PbZr\(_x\)Ti\(_{1-x}\)O\(_3\) thin films on polymer substrates. Surf Coat Technol 200:940. https://doi.org/10.1016/j.surfcoat.2005.02.041
Hubicka Z, Olejnicek J, Cada M, Virostko P, Sichova H, Deyneka A, Jastrabik L, Chvostova D, Sicha M (2005) Ba\(_x\)Sr\(_{1-x}\)TiO\(_3\) thin films deposited by RF hollow cathode plasma jet technique. Ferroelectrics 317:1. https://doi.org/10.1116/1.57129
Hubicka Z (2008) Hollow cathodes and plasma jets for thin film deposition. In: Hippler R, Kersten H, Schmidt M, Schoenbach KH (eds) Low temperature plasmas, vol 2. Wiley-VCH Verlag, Weinheim, p 715
Kuo YS, Bunshah RF, Okrent D (1986) Hot hollow cathode and its applications in vacuum coating: a concise review. J Vac Sci Technol A 4:397. https://doi.org/10.1116/1.573890
Lunk A (1990) Plasma activated physical vapour deposition by hollow cathode arc. Vacuum 41:1965. https://doi.org/10.1016/0042-207X(90)94146-H
Sobell ZC, Cavanagh AS, Boris DR, Walton SG, George SM (2021) Hollow cathode plasma electron source for low temperature deposition of cobalt films by electron-enhanced atomic layer deposition. J Vac Sci Technol, A 39:042403. https://doi.org/10.1116/6.0001033
Kapran A, Hippler R, Wulff H, Olejnicek J, Pisarikova A, Cada M, Hubicka Z (2023) Characteristics of a pulsed hollow cathode discharge operated in an Ar+O\(_2\) gas mixture and deposition of copper nickel oxide thin films. Vacuum 215:112272. https://doi.org/10.1016/j.vacuum.2023.112272
Morgner H, Neumann M, Straach S, Krug M (1998) The hollow cathode: a high-performance tool for plasma-activated deposition. Surf Coat Technol 108–109:513. https://doi.org/10.1016/S0257-8972(98)00633-1
Mendez I, Gordillo-Vazquez FJ, Herrero VJ, Tanarro I (2006) Atom and ion chemistry in low pressure hydrogen DC plasmas. J Phys Chem A 110:6060. https://doi.org/10.1021/jp057182+
Carrasco E, Tanarro I, Herrero VJ, Cernicharo J (2013) Proton transfer chains in cold plasmas of H2 with small amounts of N2. The prevalence of NH\(_4^+\). Phys Chem Chem Phys 15:1699. https://doi.org/10.1039/c2cp43438e
Carrasco E, Jimenez-Redondo M, Tanarro I, Herrero VJ (2011) Neutral and ion chemistry in low pressure dc plasmas of H2/N2 mixtures: routes for the efficient production of NH3 and NH4. Phys Chem Chem Phys 13:19561. https://doi.org/10.1039/c1cp22284h
Usmanov DT, Chen LC, Hiraoka K, Wada H, Nonami H, Yamabe S (2016) Mass spectrometric monitoring of oxidation of aliphatic C6–C8 hydrocarbons and ethanol in low pressure oxygen and air plasmas. J Mass Spectrom 51:1187. https://doi.org/10.1002/jms.3890
de los Arcos T, Domingo C, Herrero VJ, Sanz MM, Schulz A, Tanarro I (1998) Diagnostics and kinetic modeling of a Hollow Cathode N\(_2\)O discharge. J Phys Chem A 102:6282. https://doi.org/10.1021/jp981749n
Tezani L, Pessoa RS, Maciel HS, Petraconi G (2014) Chemistry studies of SF\(_6\)/CF\(_4\), SF\(_6\)/O\(_2\) and CF\(_4\)/O\(_2\) gas phase during hollow cathode reactive ion etching plasma. Vacuum 106:64. https://doi.org/10.1016/j.vacuum.2014.03.003
Jimenez-Redondo M, Carrasco E, Herrero VJ, Tanarro I (2015) Chemistry in glow discharges of H\(_2\)/O\(_2\) mixtures: diagnostics and modelling. Plasma Sources Sci Technol 24:015029. https://doi.org/10.1088/0963-0252/24/1/015029
Glarborg P, Miller JA, Ruscic B, Klippenstein SJ (2018) Modeling nitrogen chemistry in combustion. Prog Energy Combust Sci 67:31. https://doi.org/10.1016/j.pecs.2018.01.002
Gholami F, Tomas M, Gholami Z, Vakili M (2020) Technologies for the nitrogen oxides reduction from flue gas: a review. Sci Total Environ 714:136712. https://doi.org/10.1016/j.scitotenv.2020.136712
Dors M, Mizeraczyk J (1996) Conversion of nitrogen oxides in N\(_2\):O\(_2\):CO\(_2\) and N\(_2\):O\(_2\):CO\(_2\):NO\(_2\) mixtures subjected to a dc corona discharge. Czech J Phys 46:943. https://doi.org/10.1007/BF01795143
Siebers DL, Caton JA (1990) Removal of nitric oxide from exhaust gas with cyanuric acid. Combust Flame 79:31. https://doi.org/10.1016/0010-2180(90)90086-7
Rajanikanth BS, Rout S (2001) Studies on nitric oxide removal in simulated gas compositions under plasma-dielectric/catalytic discharges. Fuel Process Technol 74(3):177. https://doi.org/10.1016/S0378-3820(01)00238-7
Newnham DA, Rodger CJ, Marsh DR, Hervig ME, Clilverd MA (2020) Spatial distributions of nitric oxide in the antarctic wintertime middle atmosphere during geomagnetic storms. J Geophys Res Space Phys 125:846. https://doi.org/10.1029/2020JA027846
Gupta KJ, Kaladhar VC, Fitzpatrick TB, Fernie AR, Møller 4 IM, Loake GJ, (2022) Nitric oxide regulation of plant metabolism. Mol Plant 15:228. https://doi.org/10.1016/j.molp.2021.12.012
Astier J, Gross I, Durner J (2018) Nitric oxide production in plants: an update. J Exp Bot 69:3401. https://doi.org/10.1093/jxb/erx420
Shekhter AB, Serezhenkov VA, Rudenko TG, Pekshev AV, Vanin AF (2005) Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide 12:210. https://doi.org/10.1016/j.niox.2005.03.004
Coulter JA, McCarthy HO, Xiang J, Roedl W, Wagner E, Robson T, Hirst DG (2008) Nitric oxide-a novel therapeutic for cancer. Nitric Oxide 19:192. https://doi.org/10.1016/j.niox.2008.04.023
Pipenbaher N, Moeller PL, Dolinsek J, Jakobsen M, Weingartl H, Cencic A (2009) Nitric oxide (NO) production in mammalian non-tumorigenic epithelial cells of the small intestine and macrophages induced by individual strains of lactobacilli and bifidobacteria. Int Dairy J 19:166. https://doi.org/10.1016/j.idairyj.2008.09.003
Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, Mikhailidis DP (1999) Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res 43:658. https://doi.org/10.1016/S0008-6363(99)00135-2
The Nobel Prize in Physiology or Medicine 1998. NobelPrize.org. Nobel prize outreach AB 2023 (retrieved Fri. 28 Jul 2023). https://www.nobelprize.org/prizes/medicine/1998/summary/
Lancaster Jr JR (2017) A concise history of the discovery of mammalian nitric oxide (nitrogen monoxide) biogenesis. In: Ignarro LJ, Freeman BA, (Eds.) Nitric Oxide, 3rd edition, Chap. 1 (2017); https://doi.org/10.1016/b978-0-12-804273-1.00001-6
Landl NV, Korolev YD, Nekhoroshev VO, Frants OB, Argunov GA, Kasyanov VS (2022) Production of nitrogen oxides in a positive column of a glow-type discharge in air flow. Plasma Chem Plasma Process 42:1187. https://doi.org/10.1007/s11090-022-10262-2
Wang D, Namihira T (2020) Nanosecond pulsed streamer discharges: II. Physics, discharge characterization and plasma processing. Plasma Sources Sci Technol 29:023001. https://doi.org/10.1088/1361-6595/ab5bf6
Yang Q, Hu H, Chen W, Xu J, Zhang J, Wu S (2011) The main plasma chemical process of nitric oxide production by arc discharge. Plasma Sci. Technol. 13:702. https://doi.org/10.1088/1009-0630/13/6/13
Jinwoo K, Hyungyu L, Seong-Cheol H, Bae JH, Choe W, Han D, Park S, Ryu S, Park S (2023) Competitive formation of NO, NO\(_2\), and O\(_3\) in an air-flowing plasma reactor: a central role of the flow rate. Chem Eng J 468:143636. https://doi.org/10.1016/j.cej.2023.143636
Lalo C, Deson J, Cerveau C, Ben-Aim RI (1993) Air microwave-induced plasma: detection of NO in the post-discharge using an ArF laser. Plasma Chem Plasma Process 13:351. https://doi.org/10.1007/BF01466049
Wojtowicz MA, Miknis FP, Grimes RW, Smith WW, Serio MA (2000) Control of nitric oxide, nitrous oxide, and ammonia emissions using microwave plasmas. J Hazard Mater 74:81. https://doi.org/10.1016/S0304-3894(99)00200-9
Rousseau A, Dantier A, Gatilova L, Ionikh Y, Röpcke J, Tolmachevet Y (2005) On NO\(_x\) production and volatile organic compound removal in a pulsed microwave discharge in air. Plasma Sources Sci. Technol. 14: 70. https://doi.org/10.1088/0963-0252/14/1/009
Gritsinin SI, Knyazev VY, Kossyi IA, Popov NA (2006) Microwave torch as a plasmachemical generator of nitric oxides. Plasma Phys Rep 32:520. https://doi.org/10.1134/S1063780X06060092
Loenders B, Michiels R, Bogaerts A (2023) Is a catalyst always beneficial in plasma catalysis? Insights from the many physical and chemical interactions. J Energy Chem 85:501. https://doi.org/10.1016/j.jechem.2023.06.016
Hippler R, Cada M, Hubicka Z (2022) Ion energy distribution of plasma ions of a hollow cathode discharge in Ar + N\(_2\) and Ar + O\(_2\) gas mixtures. Eur Phys J D 76:214. https://doi.org/10.1140/epjd/s10053-022-00539-8
Hippler R, Cada M, Hubicka Z (2021) A positively biased external anode for energy control of plasma ions: hollow cathode and magnetron sputtering discharge. Plasma Sources Sci Technol 30:045003. https://doi.org/10.1088/1361-6595/abe0cc
Hippler R, Cada M, Hubicka Z (2021) Direct current and high power impulse magnetron sputtering discharges with a positively biased anode. J Vac Sci Technol, A 39:043007. https://doi.org/10.1116/6.0001054
Hippler R, Cada M, Stranak V, Hubicka Z, Helm CA (2017) Pressure dependence of Ar\(_2^+\), ArTi\(^+\), and Ti\(_2^+\) dimer formation in a magnetron sputtering discharge. J Phys D Appl Phys 50:445205. https://doi.org/10.1088/1361-6463/aa8b9a
Hippler R, Cada M, Stranak V, Helm CA, Hubicka Z (2019) Pressure dependence of singly and doubly charged ion formation in a HiPIMS discharge. J Appl Phys 125:013301. https://doi.org/10.1063/1.5055356
Heays AN, Kaiserova T, Rimmer PB, Knizek A, Petera L, Civis S, Juha L, Dudzak R, Krus M, Scherf M, Lammer H, Pascal R, Ferus M (2022) Nitrogen oxide production in laser-induced breakdown simulating impacts on the Hadean atmosphere. J. Geophys. Res.: Planets 127:e2021JE006842. https://doi.org/10.1029/2021JE006842
Petera L, Knizek A, Laitl V, Ferus M (2023) Decomposition of Benzene during Impacts in N\(_2\)-dominated atmospheres. Astrophys J 945:149. https://doi.org/10.3847/1538-4357/acbd48
Heays AN (2022) spectr, GitHub repository; https://github.com/aheays/spectr.git
Blechle JM, Hanna AR, Fisher ER (2017) Determination of internal temperatures within nitric oxide inductively coupled plasmas. Plasma Process Polym 14:e1700041. https://doi.org/10.1002/ppap.201700041
Yanjun D, Ding Y, Liu Y, Lan L, Peng Z (2014) Calibration-free self-absorption model for measuring nitric oxide concentration in a pulsed corona discharge. Appl Opt 53:4922. https://doi.org/10.1364/AO.53.004922
Olszewski R, Zubek M (2001) A study of electron impact excitation of the A \(^2\Sigma ^+\) state of nitric oxide in the near-threshold energy range. Chem Phys Lett 340:249. https://doi.org/10.1016/S0009-2614(01)00426-2
Ottinger Ch, Vilesova AF (1994) Intramolecular collisional transfer in NO (a \(^4\Pi\)\(\rightarrow\) B \(^2\Pi\), b \(^4\Sigma ^-\)): Gateway-type, resonant versus direct, nonresonant mechanisms. J Chem Phys 100:1805. https://doi.org/10.1063/1.466533
Navratil Z, Trunec D, Smid R, Lazar L (2006) A software for optical emission spectroscopy–problem formulation and application to plasma diagnostics. Czech J Phys 56:B944. https://doi.org/10.1007/s10582-006-0308-y
Kramida A, Ralchenko Y, Reader J, and NIST ASD Team (2022) NIST Atomic Spectra Database (ver. 5.10), [Online]. Available: https://physics.nist.gov/asd [2023, August 4]. National Institute of Standards and Technology, Gaithersburg, MD; https://doi.org/10.18434/T4W30F
Piper LG, Cowles LM (1986) Einstein coefficients and transition moment variation for the NO(A \(^2\Sigma ^+\) - X \(^2\Pi\)) transition. J Chem Phys 85:2419–2422. https://doi.org/10.1063/1.451098
Laane J, Ohlsen JR (1980) Characterization of nitrogen oxides by vibrational spectroscopy. Prog Inorg Chem 27:465. https://doi.org/10.1002/9780470166284.ch6
Guerra V, Tejero-del-Caz A, Pintassilgo CD, Alves LL (2019) Modelling N\(_2\)-O\(_2\) plasmas: volume and surface kinetics. Plasma Sources Sci Technol 28:073001. https://doi.org/10.1088/1361-6595/ab252c
Patil BS, Wang Q, Hessel V, Lang J (2015) Plasma N\(_2\)-fixation: 1900–2014. Catal Today 256:49. https://doi.org/10.1016/j.cattod.2015.05.005
Stefanovic I, Bibinov NK, Deryugin AA, Vinogradov IP, Napartovich AP, Wiesemann K (2001) Kinetics of ozone and nitric oxides in dielectric barrier discharges in O\(_2\)/NO\(_x\) and N\(_2\)/O\(_2\)/NO\(_x\) mixtures. Plasma Sources Sci Technol 10:406. https://doi.org/10.1088/0963-0252/10/3/303
Rouwenhorst KHR, Jardali F, Bogaerts A, Lefferts L (2021) From the Birkeland-Eyde process towards energy-efficient plasma-based NO\(_x\) synthesis: a techno-economic analysis. Energy Environ Sci 14:2520. https://doi.org/10.1039/D0EE03763J
Rouwenhorst KHR, Engelmann Y, van K (2020) ‘t Veer, R.S. Postma, A. Bogaerts, L. Lefferts, Plasma-driven catalysis: green ammonia synthesis with intermittent electricity, Green Chemistry; https://doi.org/10.1039/D0GC02058C
Rao X, Matveev IB, Lee T (2009) Nitric oxide formation in a premixed flame with high-level plasma energy coupling. IEEE Trans Plasma Sci 37:2303. https://doi.org/10.1109/TPS.2009.2034007
Britun N, Gamaleev V, Hori M (2021) Evidence of near-the-limit energy cost NO formation in atmospheric spark discharge. Plasma Sources Sci Technol 30:08LT02. https://doi.org/10.1088/1361-6595/ac12bf
Malik MA (2016) Nitric oxide production by high voltage electrical discharges for medical uses: a review. Plasma Chem Plasma Process 36:737. https://doi.org/10.1007/s11090-016-9698-1
Kossyi IA, Kostinsky YA, Matveyev A, Silakov VP (1992) Kinetic scheme of the nonequilibrium discharge in nitrogen-oxygenmixtures. Plasma Sources Sci Technol 1:207. https://doi.org/10.1088/0963-0252/1/3/011
Van Gaens W, Iseni S, Schmidt-Bleker A, Weltmann K-D, Reuter S, Bogaerts A (2015) Numerical analysis of the effect of nitrogen and oxygen admixtures on the chemistry of an argon plasma jet operating at atmospheric pressure. New J Phys 17:033003. https://doi.org/10.1088/1367-2630/17/3/033003
Zhu X, Zheng C, Gao X, Shen X, Wang Z, Luo Z, Cen K (2014) Experimental study of NO\(_2\) reduction in N\(_2\)/Ar and O\(_2\)/Ar mixtures by pulsed corona discharge. J Environ Sci 26:2249. https://doi.org/10.1016/j.jes.2014.09.010
Acknowledgements
The work was supported by Project No. 21-04477 S of the Czech Science Foundation.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
RH planned the study, carried out the research, and wrote the draft of the paper, MC and ZH organized the study and the funding and designed the reactor, AK and MF carried out the FTIR measurements and analyzed the results. All authors contributed to the final results and to the writing of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hippler, R., Cada, M., Knizek, A. et al. A Pulsed Hollow Cathode Discharge Operated in an Ar/N\(_2\)/O\(_2\) Gas Mixture and the Formation of Nitric Oxide. Plasma Chem Plasma Process 44, 1053–1068 (2024). https://doi.org/10.1007/s11090-024-10450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-024-10450-2