Abstract
The formation of carbon monoxide, oxygen and ozone in a barrier corona discharge (BCD) operating in pure carbon dioxide (CO2) and binary mixtures of CO2 and argon is studied. The asymmetric electrode configuration of the BCDs allows plasma operation at pressures exceeding 1 atm, up to 6 bar, at moderate high-voltage amplitudes below 15 kV. Charge–voltage plots and an equivalent circuit model are employed to characterize the electrical parameters at different pressures and gas compositions. Depending on these conditions and the voltage amplitude, full or partial coverage of the electrodes with plasma is obtained. The existence of an optimum pressure for power dissipation for each given operation voltage amplitude and gas composition can be confirmed and explained by the equivalent circuit model. Increasing the CO2 concentration in the working gas increases the mean reduced electric field strength E/N while pressure reduces it in the BCD. The CO2 conversion shows a maximum efficiency of about 4% at 1.5 bar for the gas mixture Ar/CO2 = 1:1 and a voltage amplitude of about 10 kV. The calculation of thermodynamic equilibrium parameters reveals that a relatively small increase in pressure can affect both, the equilibrium parameters and the reaction rates. As a result, the specific required energy for the reaction (\(\Delta \mathrm{H}/\mathrm{SEI}\)) shows an optimum, but only 8% of the electrical input energy is spent for CO2 dissociation at these optimum conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mitigation of greenhouse gases has become one of the most challenging issues in the world today [1]. Carbon dioxide (CO2) contributes up to 65% of the greenhouse emissions produced in about 50 Gt yearly from anthropogenic activities. The increase of greenhouse gas concentrations in earth atmosphere is responsible for global warming [2]. In consequence, finding solutions for CO2 reduction is a priority research topic [3]. On the other hand, carbon monoxide (CO), a likely product of CO2 decomposition, can be considered a valuable chemical feedstock and enable Power-to-X technologies [4, 5].
The major challenge of converting CO2 is to overcome the high stability of the double bonds in this linear molecule (O=C=O). This requires thermodynamically 2.9 eV/molecule (279.8 kJ/mol) [6]. The existing methods for CO2 conversion are steam methane reforming [7], steam methane/oxygen reforming [8], partial oxidation [9], partial catalytic oxidation [10], auto-thermal reforming [11], CO2 hydrogenation by reverse water gas shift reaction [12], hydrogenation to methanol [13] and CH4 [14], reduction of CO2 with H2O [15], and other biochemical [16], photochemical [17] and electrochemical [18] approaches. Plasma-assisted CO2 conversion would allow the transformation of intermittent electrical energy from renewable sources into fuels or chemicals (Power-to-X) [19]. The applicability of non-thermal plasma to convert CO2 has been studied extensively [20,21,22,23]. Due to its energetic electrons (1–10 eV), CO2 conversion is possible without heating the entire gas to more than thousand K [20]. Different plasma reactors and configurations, e.g. gliding arcs [24], microwave [25,26,27,28] and radio frequency discharges [29], glow discharges [30], nano-/microsecond pulsed discharges [31, 32], dielectric barrier discharges (DBDs) [33,34,35], and corona discharges [36, 37] have been studied. Among them, DBDs have a simple design and easy construction with low cost materials and operate with common AC power supplies. DBDs already showed their ability for scale up in high industrial gas throughputs up to several hundred standard cubic meters per hour [23]. However, the energy efficiency of CO2 conversion in DBDs is lower (< 15%) compared to microwave discharges and gliding arcs [20].
The effects of different operation parameters on DBD’s conversion efficacy have been widely studied. These parameters are the specific energy input (SEI, which is determined by discharge power and gas flow rate [38]); reactor geometry (e.g. discharge gap width, dielectric material and its thickness) [39]; dilution of CO2 in other gases (e.g. nitrogen, argon, helium) [40, 41]; gas temperature [42, 43]; application of external magnetic fields [44], change of the pressure [45]. While Ponduri et al. [46]. concluded that there should be no significant effect by pressure in plane parallel volume DBDs, Bak et al. reported a higher conversion rate and lower energy efficiency of CO2 splitting by increasing the pressure (from 2.36 to 5.08 atm) in nanosecond-pulsed DBDs [47]. Belov et al. reported that pressure increases not only tune the discharge current of the single microdischarges forming the plasma (and possibly the underlying plasma chemistry), but also triggers CO2 conversion efficiency [48]. DBDs operating at overpressures in air or oxygen [49,50,51] demonstrated the existence of an optimal pressure for ozone synthesis, namely 1.4 bar in air [51] and 1.5–2 bar for oxygen [50]. Furthermore, studies on nanosecond pulsed surface DBDs operated at pressures between 1 and 6 bar in air gave evidence that the discharge dynamics changed with the pressure [52, 53]. It was also demonstrated that overpressure could influence plasma-assisted ignition systems [54] and the vibrational distribution function in pure N2 discharges. There are a few works on CO2-containing discharges, mostly on CO2 reforming with methane and hydrocarbons synthesis from syngas (CO + H2) (Fischer–Tropsch process) using non-thermal discharges at very high pressures [55, 56]. Recently, Hosseini Rad et al. demonstrated the enhancement of the energy yield for CO formation by pressure increase from 1 to 2 bar in an argon-CO2 gas mixture (ratio Ar:CO2 = 4:1) [57]. This study aims to develop a barrier discharge reactor for CO2 conversion at pressures higher than 2 bar, but lower argon concentration in the gas.
Barrier corona discharges (BCDs) are a combination of a one-sided DBD and corona discharge, i.e. is the bare metal electrode that contains one or more sharpened pins to enhance the electric field strength. BCDs are employed in many applications such as surface modification and exhaust gas treatment [58]. Beside the study of such applications, there are numerous investigations in single filament BCDs, i.e. between one metal pin and a dielectric-covered plane or semi-spherical electrode, see e.g. [59, 60]. The enhanced electric fields strength around the pins reduces the voltage amplitude required for breakdown. Since a higher voltage amplitude is expected for increasing pressure due to Paschen’s law (for gap times distance g × p > 2 bar mm), a pin or brush electrode should enable to operate plasma at higher pressure with still moderate high voltage amplitudes.
To explore the effect of pressure increase (p > 1 bar) in a BCDs the electrical discharge parameters, such as the discharge voltage, plasma spreading over reactor volume and discharge power are studied by applying an equivalent circuit model. Furthermore, the influence of pressure on the mean reduced electric field strength E/N is of high interest, as this similarity parameter relates to the energy gained by electrons between two collisions. Thus, it determines the electron energy distribution function (EEDF). The EEDF determines the rate coefficients of the electronic processes and thus, has an impact on the plasma chemistry. For example, a lower E/N (< 100 Td in pure CO2) is supposed to enhance the contribution of vibrational excited molecules to CO2 dissociation, while a higher E/N (> 100 Td) favors direct electronic dissociation, which has a higher threshold energy [61]. In DBDs, the mean E/N is in the range 100–200 Td, thus direct electronic dissociation dominates. However, the question of whether E/N can be influenced and whether this has an impact on conversion in DBDs remains open and will be addressed in this contribution.
Materials and Methods
Experimental Setup
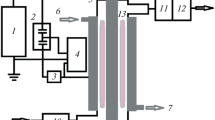
The conversion of CO2 was studied in a stainless steel, pressure-tight reactor. The electrodes were arranged in an asymmetric coaxial configuration. The high voltage electrode, as schematically shown in Fig. 1a, was a steel brush consisting of a large number of sharp metallic pins. A glass tube with a copper tape attached to the outer surface was used as the dielectric and grounded electrode, respectively. Table 1 contains the main parameters and its values. The inner diameter of the glass tube and diameter of the brush electrode resulted in discharge gap of about 2 mm.
The pressure-tight chamber with the electrode arrangement was equipped with a front quartz glass window for side observation of the BCD. The reactor was further equipped with two lateral connections for gas inlet and outlet, as illustrated in the experimental setup scheme in Fig. 2. The total gas flow through the reactor was set to 200 sccm by mass flow controllers (Bronkhorst, EL-FLOW Prestige). Gas cylinders of Ar (AirLiquide, AlphaGaz 1, purity 99.999 vol.%) and CO2 (AirLiquide, Lasal 2, purity 99.995 vol.%) were used. Different gas compositions (Ar/CO2) were supplied by setting the individual gas flows. A pressure valve regulator (Swagelok, Serie BP) was used to adjust the pressure inside the chamber, which could be varied from 1 to 6 bar. When the pressure valve was open the system flushed against the actual pressure in the laboratory which was p = (1015 ± 37) mbar during the period of our measurements. Since the mean value is near to 1013.25 mbar = 1 atm were refer to this condition as “atmospheric” or “1 atm” in the text and figures.
Before each experiment, the chamber was purged with gas for 15 min. The pressure inside the chamber was monitored during the experiments using an analogue pressure gauge (Riegler standard mano, 110.46-KDE).
The discharge was operated by a sinusoidal power supply (Chroma, Mod. 61604) connected to a high voltage transformer (Bremer Transformatoren GmbH, output 0–9 kVrms). The voltage amplitude was up to 24 kVpp while a frequency of 1 kHz was used in all experiments. Electrical measurements were performed with fast voltage (Tektronix, P6015A) and current probes (Pearson, 2877) at the high voltage (HV) and grounded electrode, respectively. Both signals were recorded with a digital phosphor oscilloscope (Tektronix, DPO4104B, 1 GHz bandwidth, 5 GS/s). Furthermore, the charge was recorded by a 1:10 voltage probe across a measuring capacitor (0.47 μF) between the copper electrode and its grounding point.
The outlet gas was analyzed with an ozone analyzer (BMT, 963) installed about 50 cm after the pressure valve and a Micro Gas Chromatograph (Inficon GmbH, 3000 Micro GC). The ozone analyzer also destroys all ozone. The sampling point of the Micro GC was about 8 m behind the ozone analyzer outlet. Micro GC was equipped with a thermal conductivity detector to measure the CO2 and CO concentrations and has been calibrated for each gaseous component using standard gas cylinders.
Evaluation of Chemical Conversion
To examine the efficiency of the BCD on CO2 dissociation the degree of CO2 conversion (χ), CO production, energy efficiency (η), and energy yield (EY) were determined. The concentration of CO, [CO], is measured by the Micro GC directly based on the device calibration, while the conversion of CO2 is calculated by Eq. (1).
where \({\mathrm{n}}_{{CO}_{2}}^{in}\) and \({\mathrm{n}}_{{CO}_{2}}^{out}\) are the molar amounts of CO2 in the mixture before and after the reactor, respectively by assuming that the total amount of argon and CO2 before the reactor is equal to 1 mol. The molar amounts \({\mathrm{n}}_{{\text{CO}}_{2}}^{in}=1/100\times ({\left[{\text{CO}}_{2}\right]}^{in})\) and \({\mathrm{n}}_{{\text{CO}}_{2}}^{out}=1/100\times {\left[{\text{CO}}_{2}\right]}^{out}\times {\left[{\text{CO}}_{2}\right]}^{in}/({\left[{\text{CO}}_{2}\right]}^{out}+{\left[{\text{CO}}\right]}^{out})\) are derived from the measured concentration \(\left[{\text{CO}}\right]\) and \(\left[{\text{CO}}_{2}\right]\) as calculated by the balances of the amounts being formed. This procedure considers the gas expansion by CO2 splitting to CO, O2 and O3 similar as described in [62].
In the case of argon dilution, the effective conversion should be considered as follows:
The CO2 concentration in the gas mixture was varied from 10 vol.% to 100 vol.%, i.e. the molar fraction \({\text{x}}_{{{\text{CO}}_{2} }} = 0.{1 } \ldots { 1}.0\).
The specific energy input SEI is determined by the discharge power and the gas flow rate (at standard temperature and pressure).
Apart from the CO2 conversion, energy efficiency is the most important index to evaluate and compare the performance of the BCD with other processes and reactors. By considering the reaction enthalpies of CO2 decomposition to CO and 0.5 O2 (ΔrH = 279.8 kJ/mol), the energy efficiency is defined as:
In Eq. 4 χeff is related to the reaction CO2 → CO + 0.5O2 and therefore, \({\text{n}}_{{{\text{CO}}_{{2}} }}^{in} - {\text{n}}_{{CO_{2} }}^{out} = {\text{n}}_{{{\text{CO}}}}\). Furthermore, the energy yield of CO production, as defined in the following Eq. (5), was calculated to evaluate the efficacy regarding the formation of the target molecule CO
where \({\text{n}}_{\text{CO}}\) and \({\text{x}}_{\text{CO}}\) are the molar amount and fraction of CO being produced, MW is the molar mass of CO, and Vm is the molar volume. The second term accounts for the gas expansion effect (1.5 molecules formed from one CO2 molecule). It neglects the formation of ozone, but this acceptable since most of the oxygen atoms form molecular oxygen.
Thermodynamic Considerations of CO2 Splitting
The input data for calculating thermodynamic parameters were extracted with Factsage software (version 6.1) for all species based on their heat capacity and for the reaction temperature at T = 300–320 K. The thermodynamic considerations are characterized by the reaction variables ΔrG°, ΔrS° and reaction heat ΔrH°. The relation between them is given by:
The individual standard reaction values are formed from the standard formation values:
\({n}_{i}\) is the molar amount and \({\nu }_{i}\) the stoichiometric coefficient of each compound of the reaction.
At ambient conditions, CO2 splitting is an endergonic and nonspontaneous (Δrg < 0) reaction. It can only take place by adding reversible work Δrg and reversible heat TΔrs. Here Δrg and Δrs are, in contrast to ΔrG and ΔrS, not the molar values, but refer to any chemical conversion.
In this paper, only the gas compositions upstream and downstream of the reactor were used for the thermodynamic considerations. The calculations were carried out by taking into account the reaction temperature, molar amount, and partial pressure of reactants and products, while elementary processes and individual reaction steps like vibrational and electronic excitations are not taken into account.
In this paper the Gibbs free energy of reaction (Δrg), is calculated by the initial amount of 1 mol Ar and CO2 and the difference between the respective sums of the formation Gibbs free energies Δrgi of CO, O2, O3, Ar and CO2 and their molar amounts and partial pressures before (ni,0, pi,0) and after (ni, pi) the reactor:
The indices r and f correspond to the reaction and formation of each species, and R and T represent the molar constant for gases (R = 8.31 J K−1⋅mol−1) and gas temperature, respectively. To measure the gas temperature inside the plasma zone in a pressure-tight chamber is problematic. However, the temperature measured at the surface of the electrode by an IR thermometer was taken for an estimation.
Results and Discussion
The results section offers a description of the electrical, chemical, and thermodynamic characteristics of CO2 plasma discharge under various gas mixtures and pressures.
Interpretation of Electrical Measurements
The measured voltage U(t) and charge Q(t) signals are used to elucidate the discharge voltage \({U}_{D}\), the mean reduced electric field strength \(E/N\) and the coverage fraction of the electrode surface \(\beta\). The charge–voltage plots (Q–V plots) such as shown in Fig. 3b show the form of a parallelogram. Its area is the energy dissipated into the plasma per HV cycle. Furthermore, the minimum sustaining voltage \({U}_{min}\) and the capacities of the discharge arrangement can be elucidated from it, as given in the figure.
The interpretation of the Q–V plots is based on the equivalent circuit in Fig. 3a which was introduced by Peeters and van de Sanden [63]. The simplest equivalent circuit consists of two capacitances in linear arrangement. The first, \({C}_{diel}\), represents the dielectric barrier, the second, \({C}_{gap}\), the discharge gap. Both capacities form the total capacity \(, {C}_{cell}\). When the applied voltage amplitude \({U}_{pp}\) is below the threshold \({U}_{min}\), the voltage across the discharge gap is not sufficient to cause electrical breakdown; and the Q–V plot will consist of a line with the slope \({C}_{cell}\). If full breakdown happens for \({U}_{pp}{\gg U}_{min}\), the parallelogram will form and its sides have the slopes \({C}_{cell}\) and \({C}_{diel}\).
However, in our BCD reactor, the discharge appears non-uniform in the discharge gap. The plasma itself is filamentary, i.e., consisting of many individual discharge channels, which develop between the pin electrode and the inner surface of the dielectric; and spread over the surface [59, 64]. The diameter of the microdischarge foot on the dielectric is much higher than the diameter of the streamer in the volume (some mm vs. 100 µm). The microdischarges do not appear simultaneously and do not penetrate the full volume between the electrodes or cover the entire dielectric surface. From a more microscopic point of view, the pins of the brush do not have identical length, shapes and orientations or can be contaminated by dust or other surface coverage. Consequently, there are locations with a lower or a higher voltage threshold, see Fig. 1b. Therefore, at low overvoltage, the electric field strength is not sufficiently high at all pins and microdischarges will only appear at the longest pins. When the applied voltage is high enough, the electric field strength is sufficient at more pins, the plasma will appear in the entire discharge region volume. This somewhat less defined breakdown voltage manifests in the lower right and upper left corner of the Q–V parallelogram. They are not sharp, but smooth. Furthermore, the steepest slope of the parallelogram, named effective dielectric capacity \({\zeta }_{diel}\), will be lower than \({C}_{diel}\) in case of partial surface discharging. The equivalent circuit in Fig. 3a considers this by dividing the electrode area into a non-discharging (characterized by parameter \(\alpha\)) and a discharging part (parameter \(\beta\)) with \(\alpha +\beta =1\). These parameters are calculated as follows.
The effective dielectric capacitance is then calculated as \({\zeta }_{diel}=\alpha {C}_{cell}+\beta {C}_{diel}\).
As higher the voltage amplitude \({U}_{pp}\) as lower is the value of \(\alpha\). For full coverage (obtained at \({U}_{pp}\gg {U}_{min}\)) we see \(\beta \to 1\) and \(\alpha =0\). Figure 4b shows this for a BCD in pure CO2 at 1 bar. While \({\zeta }_{diel}\) increases with the voltage amplitude, the values of \({C}_{gap}\) and \({C}_{cell}\) remain nearly constant (\({C}_{cell}\) at 23 pF, nearly the same value is measured by an LCR-meter), see Fig. 4a. Thus, the passive phase of the Q–V plot is not affected by \({U}_{pp}\). It only depends on the reactor geometry. It should be noted that there is a slight discrepancy in the measured values of ζdiel obtained for the positive and negative discharging phases, but this can be explained by the asymmetry of the reactor. The capacity of the dielectric tube is calculated by using the equation
for cylindrical capacitors with inner and outer radius of \({d}_{in}/2\) and \({d}_{out}/2\). The value of \({\zeta }_{diel}\) nearly saturates at \({C}_{diel}\), but for the highest voltage amplitude it slightly exceeds. This is due to the propagation of the discharge beyond the electrode area (so-called edge effect).
The time resolved slope of current and voltage are shown in Fig. 5. The discharge current shows the filamentary discharge behavior through the current spikes (the capacitive displacement current component is not measured by the current probe because its lower bandwidth is under 1 kHz). The measured charge and voltage as well as the obtained capacitances allow the calculation of the gap voltage \({U}_{gap}\left(t\right)\):
The result (based on average measured curves for \(U\left(t\right)\) and \(Q\left(t\right)\)) is also presented in the Fig. 5 (purple curve). As seen there, the gap voltage proceeds the applied voltage (due to the electric field of the residual surface charges) and is almost constant during the active discharge phase. The plateau value of the gap voltage is the discharge voltage \({U}_{D}\), a macroscopic or averaged discharge parameter for a DBD with several simultaneous microdischarges.
The discharge voltage \({U}_{D}\) can be determined from the Q–V plot also, either from \({U}_{min}\) or from the voltage difference \(\Delta U\) as follows [63, 65].
Thus, \({U}_{D}\) and \(\Delta U\) are not necessarily the same, in particular at low overvoltage and partial surface discharging [70]. It can be underestimated if this effect is not considered. However, as shown in Fig. 6, \({U}_{min}\) increases with voltage amplitude, \({U}_{pp}\). The calculation of \({U}_{D}\) as the voltage threshold for the discharge gap includes the actual capacities (see Fig. 4a), but shows a similar trend to that of \({U}_{min}\). For the conditions in this figure, the saturated value for the discharge voltage corresponds to an electric field strength of about 1.9 kV/mm. The lowest value of \({U}_{D}\) at 10 kVpp gives a voltage difference of 0.9 kV which corresponds to a gap width uncertainty of about \(\Delta g\)= 50 µm. However, these differences can be also due to different shapes and orientations of the pins or by impurities such as dust particles or layer on the pins.
\({U}_{D}\) determines the mean reduced electric field strength E/N [63, 65,66,67]. To calculate the values, we have to consider the cylindrical geometry.
Here \(r\) is the mean radius of the discharge gap \(r=\frac{{r}_{out}+{r}_{in}}{2}\) and NA, p are Avogadro’s number and the pressure inside the reactor. The non-SI Unit of E/N is 1 Td (Townsend), equal to 10–21 V·m2.
The energy per HV cycle E and the discharge power P can be determined from the area of the Q–V plot:
or calculated by Manley’s formula, which was developed for the characterization of ozonisers, as reported by Kogelschatz [68]. In its latter form, it includes the partial surface discharging effect. It converges into the original equation for full discharging with ζdiel = Cdiel [57, 69].
Figure 7 shows the measured power for the BCD in pure CO2 at 1 atm (black symbols). The blue line is the power calculated with the original Manley equation with ζdiel = Cdiel = 150 pF and the lowest minimum sustaining voltage of 7.7 kVpp. The red symbols are calculated power taking into account the partial surface discharging effect, i.e. ζdiel and the actual measured values of \({U}_{min}\) (see Fig. 6) are taken into Eq. (16). There is a fair agreement between the data extracted from the Q–V plot area and the calculation with the adapted Manley formula, while the original equation (blue line) overestimates the power at lower voltage amplitudes. The minimum sustaining voltage derived from the interpolation of the symbols to \(P\) = 0 W results in a higher value (9.3 kVpp) than obtained in the experiments. However, the 7.7 kVpp only represents the ignition at the longest metal pins, while at higher voltage amplitudes shorter pins also contribute to the discharge.
Influence of Gas Composition and Pressure on Plasma Operation
Figure 8a shows front photos of BCDs operating in different binary mixtures of Ar and CO2 with 10–100 vol.% of CO2 at 14 kVpp. The microdischarges attach to the tips of the brush electrode and spread over the inner surface of the dielectric tube. The corresponding discharge power is \(P=\) (6.0 ± 0.2) W in all figures. Figure 8e shows the corresponding current measurements. The filamentary character of the plasmas is resembled by the current spikes in the active discharge phases with a duration of about a quarter period of the high voltage (250 µs). The appearance of different groups of microdischarges, as obtained in the single-filament BCD arrangement in [59, 64], cannot be confirmed here. The microdischarges are distributed throughout the entire discharge volume. The discharge appears brighter as the Ar concentration increases with is also resembled in the current signals; with increasing Ar the more filaments are formed. As plotted in Fig. 8b, \({U}_{D}\) and \({U}_{min}\) increase with CO2 concentration. This is in agreement with the known Paschen curves and can be explained by the molecular nature as well as the electronegativity of CO2 [57]. Argon reduces the breakdown voltage. Thus, the value of mean E/N also increases linearly with CO2 concentration.
Effect of Argon addition on discharge morphology and electrical characteristics. a Front view images of BCD operating in different dilutions of Ar in CO2 at UPP = 14 kVpp (corresponds to P = (6.0 ± 0.2) W and SEI = (1.8 ± 0.1) kJ/L). b UD, Umin, and mean E/N versus CO2 concentration, measured at 14 kVpp. c ζdiel versus CO2 concentration at different UPP. d Power vs. UD for different UPP; lines are inverted parabolic fits based on Manley’s formula. e Temporal development of discharge current at UPP = 14 kVpp
The difference between \({U}_{D}\) and \({U}_{min}\) is smaller for full discharging, as explained with Eq. (13) due to the higher \({\zeta }_{diel}\). These values are presented in Fig. 8c and compared with the calculated value of \({C}_{diel}\) (black dashed line). The partial surface discharging is affected by both the gas composition and the applied voltage amplitude. For low CO2 concentration the discharge voltage is the lowest and even 10 kVpp are sufficient to cause full breakdown. The effective capacity exceeds the calculated \({C}_{diel}\) due to the spreading of the discharge beyond the electrode area. However, a much higher voltage amplitude of 14 kVpp is required to achieve nearly complete discharging in pure CO2, where propagation beyond the electrode area is not as pronounced as with argon.
The discharge power obtained from Q–V plots as a function of \({U}_{D}\) for different gas compositions but constant \({U}_{pp}\) show an inverted quadratic behavior (dashed lines), see Fig. 8d. These power parabolas are well depicted by the Manley Eq. (16) with \(P\propto \left({U}_{min}{U}_{pp}-{U}_{min}^{2}\right)\) and \({U}_{D}\propto {U}_{min}\), see Eq. (13). This equation also explain the shift of the maximum power to higher values with the voltage amplitude. The calculated power parabolas (dashed lines) fit better for lower \({U}_{D}\) (i.e. higher Ar) because of less pronounced partial discharging. For higher CO2 concentrations the original Manley equation overestimates the power, as obtained also in Fig. 7.
So, for a constant voltage amplitude there is an optimal gas composition, fulfilling the condition \({U}_{pp}=2{U}_{min}=2{U}_{D}\left[{\zeta }_{diel}/\left({\zeta }_{diel}-{C}_{cell}\right)\right]\), see [68]. In case of limited high voltage amplitudes, it is possible to optimize the power by the gas composition. In our case, at \({U}_{pp}\)= 10 kVpp the maximum discharge power is obtained for the gas mixture CO2:Ar = 3:7, while for 14 kVpp it is at CO2:Ar = 1:1 and for 18 kVpp at CO2:Ar = 4:1 (marked by the arrows).
First, the effect of pressure on electrical characteristics was investigated for Ar:CO2 = 1:1 at \({U}_{pp}\)=19.5 kVpp. Under these conditions, full discharging is achieved. As shown in Fig. 9a, \({U}_{D}\) and \({U}_{min}\) increase with pressure linearly, as predicted by the Paschen law (for \(g\times p\) > 2 bar × mm). Although both electric field strength E and density N depend on pressure linearly, the mean E/N decreases with pressure from 75 Td at 1 bar to about 40 Td for 4 bar. This could be explained by an offset of the voltage threshold. Extrapolating the fitting lines gives a nonzero value of \({U}_{D}\) and thus \({U}_{D}\approx {U}_{D,0}+c\times p\) (with \(c\) as a constant) and finally \(E/N\propto\) \({U}_{D,0}/p+c\). Since \({U}_{D}\) varies with pressure, the plot of power vs. \({U}_{D}\) in Fig. 9b shows the same inverted parabola as obtained for the gas composition. For the amplitude of 19.5 kVpp, the optimum pressure would be about 1.6 bar, which corresponds to \({U}_{D}\) = 3.9 kV and \({U}_{min}\) = 9.7 kVpp, fulfilling the condition \({U}_{pp}=2 {U}_{min}\). In agreement with this, the corresponding current slopes in Fig. 9c show lower discharge activity with increasing pressure. The current pulses appear weaker and less intense; the active discharge phases become shorter. The decrease in the number of microdischarges with pressure is in agreement with Belov et al. [48]. As is also evident from the corresponding Q–V plots, the transferred charge decreases with pressure.
The impact of discharge activity by increased pressure is also visible in the front photos in Fig. 10a. For 3 and 4 bar, fewer microdischarge channels are obtained than for 1 and 2 bar. The plasma power and the specific energy can be adjusted by the voltage amplitude. In Fig. 10a and b the voltage was set to obtain SEI = 2 or 3 kJ/L. Since the number of microdischarges decreases while the power remains constant, the energy per microdischarge must increase with pressure. Belov et al. also reported higher current amplitudes for higher pressures and voltage amplitudes [48]. The luminosity of the plasma correlates with the areal fraction coefficients α and β as shown in Fig. 10b. The coefficients were calculated with Eqs. (12) and (13) from the experimentally measured capacities shown in Fig. 10c. For the lowest pressure, the β coefficient slightly exceeds 1, i.e., the plasma covers little more than the entire electrode area. For pressures above 2 bar the voltage amplitude of 19.5 kVpp is not sufficient for complete discharging, and at 4 bar only 62% of the area is covered.
Based on the results of the electrical characterization, the operational parameters for the chemical investigations were chosen. The role of gas composition was first investigated at atmospheric pressure. Then, the pressure was varied in the mixture Ar:CO2 = 1:1. In these experiments, the voltage amplitude was set to values that ensure almost complete surface discharging, but kept constant, i.e., the plasma power and \(SEI\) were different, which has to be considered in the analysis. Finally, studies were completed for constant power and \(SEI\) for different selected gas compositions and pressures by adjusting the voltage amplitude.
Plasma Assisted CO2 Dissociation
Effect of Ar Dilution
Figure 11 shows power, CO2 conversion, CO production, energy efficiency, and energy yield as a function of CO2 concentration in argon (inlet concentrations of 10, 25, 50, 75 and 100 vol.%) for a constant applied voltage amplitude of 14 kVpp. At this amplitude the power shows little variation with gas composition and is almost constant at P = (6.0 ± 0.2) W (see Figs. 8d and 11a). Graphs (b) and (c) demonstrate that absolute CO2 conversion decreases drastically with addition of CO2, while effective conversion and CO concentration increase significantly. Ramarkers et al. [70] and Lindon et al. [71] discussed CO2 splitting in Ar:CO2 mixtures and found a higher absolute conversion with the addition of Ar. Schiorlin et al. also reported a higher CO concentration for CO2 mixed with Ar at a constant \(SEI\) in a coplanar DBD [34]. They explained this effect by stronger microdischarges or higher electron density and mean electron energy and, thus, a higher rate coefficient for dissociation processes.
a Discharge power (with quadratic fitting curve), b absolute CO2 conversion and CO concentration, c effective CO2 conversion, and d energy efficiency of CO2 splitting and energy yield of CO formation at atmospheric pressure by changing the CO2 concentration in the mixture with Ar at 14 kVpp (equal to SEI = (1.8 ± 0.2) kJ/L)
However, the effective CO2 conversion drops with the addition of Ar, due to the lower CO2 content in the gas mixture and the lower reaction rates. Therefore, since SEI is almost constant, energy efficiency and energy yield of CO are maximal in pure CO2, namely at 12% and 26 gCO/kWh. These values are comparable to those obtained in other DBDs in this SEI range [69]. The fact that the filaments penetrate a slightly smaller volume of the plasma reactor for higher CO2 concentrations (see Fig. 8a and e) has no consequence on efficiency. It should be mentioned that a considerable portion of the gas flows along the brush pins, thus not being penetrated by filaments. This is different from most other coaxial reactors in which the entire gas flows through the active discharge zone. Since CO formation is mainly due to electronic dissociation, which is only present in microdischarges, this is a limiting factor for the effectiveness and efficacy of the BCD reactor. From this point of view, the achieved energetic yield is promising.
Effect of Pressure Increase
As mentioned above, the pressure increase was studied in two different experimental campaigns. First, the gas mixtures Ar:CO2 = 3:1, 1:1 and 0:1 were used, while the voltage amplitude remained constant. This resulted in different plasma powers, \(SEI\) and mean \(E/N\) values (see Fig. 9). In the second set of experiments, the power and thus, \(SEI\) were kept constant by adjusting the voltage to the gas composition and pressure. It should be kept in mind that the pressure increase did not influence the \(SEI\), since the reactor was operated with the same gas flow rates (given in liters at standard pressure and temperature, Ln). In fact, the total number of gas particles exposed by the discharge increases with pressure, but is also flowing slower through the reactor enhancing the mean residence time of the gas in the plasma zone. Therefore, the calculation of \(EY\) is also not affected by pressure, as long as the gas flow rate under standard conditions is considered.
As shown in Fig. 12b, c, for Ar:CO2 = 1:1 at 19.5 kVpp, CO2 conversion and CO generation show a maximum at about 1.5 bar. The produced concentration CO at 1 bar is (15,100 ± 700) ppm, about three times higher than the produced at 4 bar with (5300 ± 400) ppm. Similarly, CO formation for the Ar:CO2 = 3:1 mixture at the same experimental conditions increases from about 10,000 ppm at 1.5 bar to 11,000 ppm at 3 bar and then decreases to 7,800 ppm at 5 bar. There is a similar inverted parabola for pure CO2, where the CO concentration decreases from about 17,500 ppm at 1 bar to 3900 ppm at 3 bar. Based on interpolation, the parabola's maximum was observed at 1 bar or even lower. Belov et al. also reported a maximum conversion at 1.5 bar in a coaxial DBD with pure CO2 [48]. However, it must be considered that the power differed significantly in these experiments, as shown in Fig. 12a, because of the significant increase in breakdown voltage with pressure. In fact, the same inverse parabolic behavior of power is obtained for chemical conversion. Consequently, \(\eta\) and \(EY\) are almost constant at (5 ± 1) % and (17 ± 2) gCO/kWh, respectively, for all selected gas compositions.
As mentioned in [72], \(SEI\) is the most crucial parameter for CO2 conversion. For \(SEI\) < 104 J/L a proportionality with CO formation and SEI is obtained. In order to keep the \(SEI\) constant, the voltage amplitude was adjusted. The absolute conversion and CO concentration for \(SEI\hspace{0.17em}\)= 2 kJ/L (\(P\hspace{0.17em}\)= 6.7 W) in Ar:CO2 = 1:1 is presented in Fig. 13a. The applied voltage amplitudes are shown at the top of the diagram. At first glance, these results do not reveal significant differences. However, \(\eta\) and \(EY\) show an optimum at 1.5 bar and then slightly decrease with the pressure; CO concentration drops from about 8,000 ppm to 6,830 ppm at 4 bar.
These results are interesting in several aspects. First, the pressure rise should increase the collision probability between CO2 molecules and energetic electrons or other reactive species. On the other hand, Le Chatelier’s principle for the gross reaction 2CO2 ↔ 2CO + O2 predicts a shift in equilibrium toward the side with the least amount of molecules, namely CO2 at higher pressure. However, neither a significantly improved efficacy of CO formation nor a decrease is obtained. In [57] a nearly threefold increase of \(EY\) was obtained in a coaxial DBD reactor by doubling the pressure to 2 bar, but in a gas mixture Ar:CO2 = 4:1. To investigate whether the role of pressure is different for the different binary Ar:CO2 gas mixtures, we studied the CO generation, CO2 conversion and EY for the different gas mixtures at 1 bar and 3 bar for a constant SEI = (1.8 ± 0.2) kJ/L by adjusting the voltage amplitude.
As shown in Fig. 14a, CO production at 1 bar exhibits an upward trend as the CO2 content increases from 10 vol.% (Ar:CO2 = 9:1) to pure CO2. A remarkable difference in CO formation is observed between 1 and 3 bar for 10 vol.% CO2 in Ar. At 3 bar, CO formation experiences a slight increase, but reaches a plateau at 11,000 ppm for all other gas composition. A lower absolute conversion, saturating at about 1% as illustrated in Fig. 14b, is achieved because of the higher CO2 concentration. For 10 vol.% CO2 in Ar, pressure enhances the energy yield by 50% to about 24 gCO/kWh. As the CO2 content increases, the pressure effect becomes smaller, and for pure CO2 it cannot be concluded that pressure has a significant effect; the energy yield remains at about 24 gCO/kWh. These findings are somewhat consistent with [57].
In Fig. 15, the results of the BCD are compared with the literature. The data for comparison were obtained in pure CO2 at atmospheric pressure in empty plane parallel volume DBDs (a–c) or coaxial DBDs (without packed bed, d-g), and a coplanar DBD (i). Higher pressure was studied in one of the works of Belov et al. (e), reference [48]. The work (h) includes the addition of argon [70]. Only papers that present CO concentrations or CO2 conversion together with a carbon balance or the selectivity of CO were considered for the figure. The approximate ranges of the literature results are visualized by the colored areas in the diagram. The experiments were operated in the power range \(P = { 1}...{ 15}0{\text{W}}\) at different gas flows and thus, the \(SEI\) spans several orders of magnitude. Therefore, similar as in [48] a double logarithmic scaling was chosen for the presentation of the data. The dashed lines represent values of constant\(EY\); its theoretical maximum was marked by the full red line. The stoichiometric limit of the gross reaction (CO2 → CO + 1/2 O2) was indicated by the horizontal full red line. Indeed, it neglects the ozone formation; but, its concentrations were much lower than for O2 (see next sub-section).
For all of the data, the values are found in an “\(EY\) corridor” between 3 and 30 g/kWh and the data obtained with the BCD for 1, 2 and 3 bar are within this corridor. So, the BCD is competitive with the planar and coaxial reactors. However, a significant impact by the presence of the pin electrodes or the pressure increase cannot be concluded from this graph. Similar as in (h) the addition of Ar reduces the \(EY\) slightly. The \(SEI\) is a sole scaling parameter like in the other experiments below about 104 J/Ln. For a higher \(SEI\) the retention time of the gas in the active discharge zone becomes important [38].
Obviously, looking at Fig. 14, the pressure impact is related to the presence of argon. According to the most recent reaction kinetic model for non-thermal plasma at atmospheric pressure in pure argon [79], the electron impact leads to ionization (R1) and excitation (R2) of argon atoms.
Among all excited argon states, its metastable excited species (denoted as Ar* with \({E}_{exc}\hspace{0.17em}\)= 11.55 eV; 11.72 eV here) play a key role in these plasmas, as they form excimers (R3) or can more easily be ionized (R4, R5). Excimers and argon ions contribute to the formation of Ar2+ ions by electron-excimer recombination (R6) and 3-body charge transfer (R7). The Ar2+ is by far the most important ion under these conditions. Both ions undergo electron–ion recombination (R8).
In DBDs in CO2, the dominant dissociation process is direct electron impact (R9). Other processes are dissociative electron attachment and electronic excitation (R10, R11). Electronic dissociation via vibrational excited levels has a smaller contribution than (R9) [38].
Electron collisions lead to electron impact ionization (R12) and the formed ion can contribute to CO formation via dissociative electron–ion recombination and ion-ion recombination (R13, R14).
The main loss mechanisms for CO molecules are 3-body collisions with oxygen atoms and collisional detachment of O− (R15, R16).
The addition of argon has a significant effect on the electron kinetics; it enhances the high-energy tail of the electron energy distribution function (EEDF) and thus, the electron temperature. This has a direct impact on the rate coefficients of the processes (R9–R12). The analysis of the excitation and dissociation of CO2 mixed in argon or helium in a radiofrequency discharge revealed significantly higher vibrational temperatures of CO2 and CO molecules as well as higher conversion efficiencies for argon [80]. This was explained by the different EEDF and more efficient creation of low-energy electrons for better vibrational excitation of CO2 in argon. Taking into account, that Ar admixture decreases the mean \(E/N\) (see Fig. 8b) a lower electron temperature and thus, lower rate of electronic dissociation should be expected. This trend is inline with the higher \(EY\) and \(\eta\) being obtained in Fig. 11.
In case of Ar/CO2 gas mixtures, charge transfer processes (R17) and (R18) effectively form CO2+ ions. The enhanced formation of CO2+ ion formation can promote CO formation via dissociative electron–ion recombination (R13) [70]. In addition, argon metastable have enough energy to dissociate CO2 (R19). Argon excimers emit VUV photons around 126 nm. The corresponding energy of the photons (9.84 eV) is sufficient for the photodissociation of CO2 (R20).
Figure 16 summarizes the processes mentioned here. As outlined by the blue arrows, the processes (R3) and (R7) are 3-body processes. Their contribution increases with increasing pressure. On the other hand, CO recombination (R15) is also a 3-body process, which would be enhanced by increasing pressure as well.
The mean \(E/N\) was found to decrease slightly with pressure in the BCD. From this, a lower electron temperature and rate coefficient for the electronic dissociation processes and higher fractional losses of energetic electrons to vibrational levels of CO2 would be expected. It can still be assumed that direct dissociation (R9) or electron–ion recombination (R14) will be predominant. Therefore, the enhancement of \(EY\) by pressure under Ar addition cannot be correlated with the trend of the mean \(E/N\). The mean \(E/N\) is not a direct measure of the reduced electric field strength in the microdischarge channel. It is known that its development is characterized by the positive streamer mechanism with local \(E/N\) values exceeding the ignition electric field strength significantly [81]. This calls into question the role of the mean \(E/N\) for the plasma chemistry. To learn more about the oxygen by-products, we also analyzed these species, as discussed in the next sub-section.
Temperature, Ozone Generation and Oxygen Balance
Indeed, the discharge properties and chemical processes depend on the reaction temperature. It influences the mean electron energy, the density in the plasma reactor and the rate coefficients [67]. In particular, ozone generation is affected by gas temperature [82]. To get an estimate of temperature, the temperature of the inner electrode surface, which is in contact with the gas passing through the reactor, was measured with an infrared pyrometer (Fluke, 561 HVAC). It increased from room temperature to around 55°C within the first 30 min of discharge operation at a power of 10 W and then remained constant.
The ozone concentration at the outlet increased significantly within the first 2 min of the experiment to about 1770 ppm (see Fig. 17a), and then decreased to about 1100 ppm within about 15 min. The time scales of heating and ozone formation are in the same range, and ozone is known not to be heat stable [83]. Switching off the plasma, the ozone concentration decayed within several minutes. Thus, the increase is related to the gas exchange and sampling, while the decrease can be associated with the temperature increase. After a constant gas temperature was reached, the ozone concentration remained almost stable.
O3 formation and O2 balance in CO2 splitting. a Temporal evaluation of O3 production from pure CO2, during 30 min plasma on at 10 W followed by a 2 min of post-discharge (plasma off). b Reactor wall temperature and [O3] concentration at SEI = (3.0 ± 0.1) kJ/L constant, and c Oxygen balance with and without considering O3 formation
To analyze in detail the effect of pressure (1–3 bar) on ozone formation, its concentration was measured after at least 10 min of discharge operation. The plasma was operated at SEI = (3.0 ± 0.1) kJ/L in pure CO2. The measured values in Fig. 17b show that more O3 was generated when the pressure increased. For control, the monitored inner electrode wall temperature is shown. Pressure did not affect it, since the same flow was used in all experiments.
Ozone (O3) is generated mainly from oxygen atoms in a 3-body reaction with molecular oxygen. The latter is formed by the recombination of the oxygen atoms from the CO2 splitting.
The rate coefficient of this reaction depends on the reaction temperature. Collision with a third body (M = CO2, CO, O2, Ar) is required to dissipate the surplus energy of the reaction between O and O2 as translational energy and for impulse balance [84]. Therefore, the reaction rate will increase with pressure, as obtained in the experiment (see Fig. 17b).
For the gross reaction 2CO2 → 2CO + O2, due to stoichiometry, the ratio \(\left[{\text{CO}}\right]/\left[{\text{O}}_{2}\right]\) should be equal to 2. Figure 17c shows significantly higher values which is related to a decrease in the O2 molecule formation due to O3 formation. Taking into account the O3 formation, i.e., forming the ratio \(\left[{\text{CO}}\right]/(\left[{\text{O}}_{2}\right]+3/2\left[{\text{O}}_{3}\right])\) the value 2 is obtained as expected and for all pressures. Therefore, significant amounts of other oxygen-containing species such as carbonates, can be excluded.
Thermodynamic Aspects of Plasma-Based CO2 Splitting
Several studies have performed thermodynamic analysis for CO2 conversion [57, 77, 84,85,86]. Equilibrium thermodynamics cannot be applied to nonthermal plasmas. This is because \({\Delta }_{r}G\hspace{0.17em}\)≠ 0 as a result of adsorption of reversible work \({\Delta }_{r}g\hspace{0.17em}\)> 0. The thermodynamic calculations in this contribution are considering the concentration of different species in the ground state before and after the plasma reactor. Figure 17 shows several trends of Gibbs free energy (reversible work, \({\Delta }_{r}g\)), reversible heat (\({T\Delta }_{r}s\)) and enthalpy (\({\Delta }_{r}h\)) of the gross reaction CO2 → CO + 0.5 O2 calculated from the measured concentrations after the BCD together with the corresponding trends of \(SEI\) and wall temperature. Ozone formation was neglected due to its relatively low amounts.
The results of tree different experimental campaigns were analyzed. Figure 17a and b illustrate the influence of the gas mixture at fixed \(SEI\) and wall temperature. Figure 17c and d refer to different pressures, but constant voltage amplitude. In Fig. 17e and f, the plasma power and \(SEI\) remained constant for the different pressures due to high voltage amplitude adaptation.
The thermodynamic calculations show the conversion of electrical energy into heat in the system. The \({\Delta }_{r}h\) is the amount of energy needed to split the corresponding amount of CO2. The difference between SEI and H is the energy loss that is converted to heat. The parameters for the chemical composition of the gases upstream and downstream of the reactor are used for the calculation of \({\Delta }_{r}h={\Delta }_{r}g+T{\Delta }_{r}s\). Therefore, this value depends directly on CO production, increases with CO2 content and decreases with pressure (compare Fig. 18b and d with Figs. 14a and 12b). The influence of the temperature is negligible. In case of Fig. 18f, where pressure exhibits independence at constant power and Ar:CO2 ratio \({\Delta }_{r}g\), \(T{\Delta }_{r}s\), and \({\Delta }_{r}h\) remain relatively constant since the CO production is also nearly constant.
Electrical input energy and wall temperature as well as thermodynamic parameters (Gibbs free energy Δrg, reversible heat TΔrs, enthalpy Δrh) for CO2 splitting using BCD at a and b atmospheric pressure and diluting Ar in the mixture at 14 kVpp equal to (1.8 ± 0.1) kJ/L, c and d in a mixture of Ar:CO2 1:1 at 19.5 kVpp and 1–4 bar and e and f at constant discharge power of (6.7 ± 0.2) W (error margins in some graphs smaller than symbol's size)
While the thermodynamic parameters are directly determined by the change in gas composition, the SEI remains independent at the same voltage (Fig. 18a). This relationship is clearly illustrated in Fig. 19. The ratio \({\Delta }_{r}h/SEI\) indicates how much of the energy used (SEI) is spent for the actual reaction. The specific energy requirement, \(SER\) (or \({\Delta }_{r}h\)) is the theoretical energy required for the conversion of CO2 to CO and O2. Figure 19 shows this fraction of energy as a function of SEI. In the case of pure CO2, it is less than 8% in the BCD experiments. Obviously, more energy is absorbed in pure CO2 for the dissociation, than in Ar/CO2 (black points versus red points). A significant part of the energy is mostly converted into heat by the argon species. In addition, \({\Delta }_{r}h/SEI\) decreases with \(SEI\) for both cases, correlating with the temperature increase as heat in the system. Therefore, from the thermodynamic point of view, it seems more promising to operate the discharge at lower SEI in pure CO2.
Theoretical specific energy requirement to specific energy input ratio, as a functions of SEI extracted from Fig. 18
Conclusion and Outlook
A barrier corona discharge (BCD) is capable to operate a discharge in CO2 at pressures above 1 bar, up to 6 bar, but with voltage amplitude less than 15 kV. Besides the field enhancement at the pin electrodes of the BCD arrangement, the addition of argon helps to reduce the required high voltage amplitude. The discharge voltage increases linearly with pressure and CO2 concentration, while the power input follows the Manley equation extended by the surface partial discharging effect caused by the non-uniform discharge gap of the BCD. The discharge power scales linearly with voltage amplitude at full discharging, but inversely parabolic with discharge voltage and thus pressure.
The BCD enabled the operation at higher pressures and CO2 fractions than in the coaxial DBD reactor studied in [57]. Increasing the pressure has no dramatic negative impact on the energy yield in pure CO2. In a similar manner as observed in [57] a higher argon addition increases the CO2 splitting efficiency with pressure, potentially due to increased 3-body collisions and the dominant role of dissociative electron–ion recombination. The mean E/N, determined from the discharge voltage, does not show a systematic influence on the chemistry and the efficacy of the CO2 splitting process. The main disadvantage of the BCD compared to coaxial DBDs is that a certain amount of the gas flows through the pin electrodes without being penetrated by plasma. This reduces the overall performance of the reactor. Avoiding this, could lead to a higher efficacy.
The comparison of the reaction enthalpy and the SEI shows that most of the energy is not used for CO production, but converted to heat. The efficiency of CO2 splitting becomes higher when the SEI is smaller and the CO2 inlet concentration increases. From a thermodynamic viewpoint the most promising operation is at lower SEI in pure CO2.
These findings advance our understanding of BCD plasma in CO2 and its potential for CO2 splitting applications, offering promising directions for future research and practical implementations in plasma chemistry. However, without a comprehensive analysis of the microdischarge parameters, the electron kinetics and the plasma chemistry in the whole, including oxygen and ozone, by simulations, it is impossible to provide a more sound explanation of the effect of pressure increase on the energy yield of argon-diluted CO2. Beside these aspects other chemical processes chemistry, e.g. the CO2 reforming of methane are planned for future research.
Data availability
The data that support the findings of the study will be openly available at the public repository INPTDAT (https://www.inptdat.de) after publication.
References
Andersson B, Wallin A (2000) Students’ understanding of the greenhouse effect, the societal consequences of reducing CO2 emissions and the problem of ozone layer depletion. J Res Sci Teach 37(10):1096–1111
Dalal RC, Allen DE (2008) Greenhouse gas fluxes from natural ecosystems. Aust J Bot 56(5):369–407
Strassburg BB et al (2020) Global priority areas for ecosystem restoration. Nature 586(7831):724–729
Dibenedetto A, Angelini A, Stufano P (2014) Use of carbon dioxide as feedstock for chemicals and fuels: homogeneous and heterogeneous catalysis. J Chem Technol Biotechnol 89(3):334–353
Aresta M (2006) Carbon dioxide reduction and uses as a chemical feedstock. Act Small Mol Organom Bioinorg Perspect. https://doi.org/10.1002/9783527609352.ch1
Vadikkeettil Y et al (2022) Plasma assisted decomposition and reforming of greenhouse gases: a review of current status and emerging trends. Renew Sustain Energy Rev 161:112343
Hajjaji N et al (2012) Exergy analysis: An efficient tool for understanding and improving hydrogen production via the steam methane reforming process. Energy Policy 42:392–399
Tjatjopoulos GJ, Vasalos IA (1998) Feasibility analysis of ternary feed mixtures of methane with oxygen, steam, and carbon dioxide for the production of methanol synthesis gas. Ind Eng Chem Res 37(4):1410–1421
Ashcroft A, Cheetham AK, Green M (1991) Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 352(6332):225–226
Lago R et al (1997) Partial oxidation of methane to synthesis gas using lncoo3perovskites as catalyst precursors. J Catal 167(1):198–209
Ortiz M et al (2011) Optimization of hydrogen production by Chemical-Looping auto-thermal Reforming working with Ni-based oxygen-carriers. Int J Hydrog Energy 36(16):9663–9672
Ayodele OB (2017) Eliminating reverse water gas shift reaction in CO2 hydrogenation to primary oxygenates over MFI-type zeolite supported Cu/ZnO nanocatalysts. J CO Util 20:368–377
Bahruji H et al (2016) Pd/ZnO catalysts for direct CO2 hydrogenation to methanol. J Catal 343:133–146
Meshkini Far R et al (2018) CO2 hydrogenation into CH4 over Ni–Fe catalysts. Funct Mater Lett 11(03):1850057
Mori K, Yamashita H, Anpo M (2012) Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv 2(8):3165–3172
Woolerton TW et al (2011) CO2 photoreduction at enzyme-modified metal oxide nanoparticles. Energy Environ Sci 4(7):2393–2399
Fu J et al (2020) Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 32:222–243
Lim RJ et al (2014) A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal Today 233:169–180
Yin Y et al (2021) CO2 conversion by plasma: how to get efficient CO2 conversion and high energy efficiency. Phys Chem Chem Phys 23(13):7974–7987
Snoeckx R, Bogaerts A (2017) Plasma technology—a novel solution for CO2 conversion? Chem Soc Rev 46(19):5805–5863
Lebouvier A et al (2013) Assessment of carbon dioxide dissociation as a new route for syngas production: a comparative review and potential of plasma-based technologies. Energy Fuels 27(5):2712–2722
Chung WC, Chang MB (2016) Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew Sustain Energy Rev 62:13–31
Brandenburg R et al (2019) White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process Polym 16(1):1700238
Nagassou D et al (2020) Decomposition of CO2 in a solar-gliding arc plasma reactor: effects of water, nitrogen, methane, and process optimization. J CO2 Util 38:39–48
Bongers W et al (2017) Plasma-driven dissociation of CO2 for fuel synthesis. Plasma Process Polym 14(6):1600126
Britun N, Godfroid T, Snyders R (2020) Insights into CO2 conversion in pulsed microwave plasma using optical spectroscopy. J CO2 Util 41:101239
Falcinelli S (2020) Fuel production from waste CO2 using renewable energies. Catal Today 348:95–101
Mohsenian S et al (2019) Carbon dioxide conversion by solar-enhanced microwave plasma: effect of specific power and argon/nitrogen carrier gases. J CO2 Util 34:725–732
Kano M, Satoh G, Iizuka S (2012) Reforming of carbon dioxide to methane and methanol by electric impulse low-pressure discharge with hydrogen [hydrogenat]. Plasma Chem Plasma Process 32(2):177–185
Trenchev G et al (2019) Atmospheric pressure glow discharge for CO2 conversion: model-based exploration of the optimum reactor configuration. Chem Eng J 362:830–841
Ghorbanzadeh AM, Matin NS (2005) Methane conversion to hydrogen and higher hydrocarbons by double pulsed glow discharge. Plasma Chem Plasma Process 25(1):19–29
Chun YN, Kim SW, Song HO (2004) Steam plasma reforming of biogas by non-thermal pulsed discharge. Korean J Chem Eng 21(3):670–675
Ray D, Saha R, Subrahmanyam C (2017) DBD plasma assisted CO2 decomposition: influence of diluent gases. Catalysts 7(9):244
Schiorlin M, Klink R, Brandenburg R (2016) Carbon dioxide conversion by means of coplanar dielectric barrier discharges. Eur Phys J Appl Phys 75(2):24704
Ozkan A (2016) CO2 splitting in a dielectric barrier discharge plasma: understanding of physical and chemical aspects. Thesis
Horvath G, Skalny JD, Mason NJ (2008) FTIR study of decomposition of carbon dioxide in dc corona discharges. J Phys D-Appl Phys 41(22):225207
Li M-W et al (2006) Effects of catalysts in carbon dioxide reforming of methane via corona plasma reactions. Energy Fuels 20(3):1033–1038
Aerts R, Somers W, Bogaerts A (2015) Carbon dioxide splitting in a dielectric barrier discharge plasma: a combined experimental and computational study. Chemsuschem 8(4):702–716
Mei D, Tu X (2017) Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. J CO Util 19:68–78
Li S-Z et al (2006) Comparison of atmospheric-pressure helium and argon plasmas generated by capacitively coupled radio-frequency discharge. Phys Plasmas 13(9):093503
Moss M et al (2017) An investigation of CO2 splitting using nanosecond pulsed corona discharge: effect of argon addition on CO2 conversion and energy efficiency. Plasma Sources Sci Technol 26(3):035009
Lu N et al (2018) CO2 conversion in non-thermal plasma and plasma/g-C3N4 catalyst hybrid processes. J Phys D Appl Phys 51(9):094001
Paulussen S et al (2010) Conversion of carbon dioxide to value-added chemicals in atmospheric pressure dielectric barrier discharges. Plasma Sources Sci Technol 19(3):034015
Li L et al (2020) Magnetically enhanced gliding arc discharge for CO2 activation. J CO2 Util 35:28–37
Kano M, Satoh G, Iizuka S (2012) Reforming of carbon dioxide to methane and methanol by electric impulse low-pressure discharge with hydrogen. Plasma Chem Plasma Process 32(2):177–185
Ponduri S et al (2016) Fluid modelling of CO2 dissociation in a dielectric barrier discharge. J Appl Phys 119(9):16
Bak MS, Im S-K, Cappelli M (2015) Nanosecond-pulsed discharge plasma splitting of carbon dioxide. IEEE Trans Plasma Sci 43(4):1002–1007
Belov I, Paulussen S, Bogaerts A (2017) Pressure as an additional control handle for non-thermal atmospheric plasma processes. Plasma Process Polym 14(11):1700046
Boonduang S et al (2012) Effect of oxygen pressure and flow rate on electrical characteristic and ozone concentration of a cylinder-cylinder DBD ozone generator. Procedia Eng 32:936–942
Seok DC et al (2015) Optimizing factors on high concentration of ozone production with dielectric barrier discharge. Ozone Sci Eng 37(3):221–226
Yuan D et al (2016) Ozone production in parallel multichannel dielectric barrier discharge from oxygen and air: the influence of gas pressure. J Phys D Appl Phys 49(45):455203
Wei B et al (2019) SDBD based plasma anti-icing: a stream-wise plasma heat knife configuration and criteria energy analysis. Int J Heat Mass Transf 138:163–172
Stepanyan S et al (2014) A nanosecond surface dielectric barrier discharge in air at high pressures and different polarities of applied pulses: transition to filamentary mode. Plasma Sources Sci Technol 23(4):045003
Pancheshnyi SV et al (2006) Ignition of propane–air mixtures by a repetitively pulsed nanosecond discharge. IEEE Trans Plasma Sci 34(6):2478–2487
Eliasson B et al (1998) Hydrogenation of carbon dioxide to methanol with a discharge-activated catalyst. Ind Eng Chem Res 37(8):3350–3357
Iwarere S et al (2014) Hydrocarbons synthesis from syngas by very high pressure plasma. Chem Eng J 241:1–8
Rad RH et al (2023) Enhancement of CO2 splitting in a coaxial dielectric barrier discharge by pressure increase, packed bed and catalyst addition. Chem Eng J 456:141072
Brandenburg R (2017) Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol 26(5):053001
Jahanbakhsh S, Brüser V, Brandenburg R (2018) Single microdischarges in a barrier corona arrangement with an anodic metal pin: Discharge characteristics in subsequent breakdowns. Plasma Sources Sci Technol 27(11):115011
Akishev YS et al (2003) One-atmosphere argon dielectric-barrier corona discharge as an effective source of cold plasma for the treatment of polymer films and fabrics. High Energy Chem 37(5):286–291
Pietanza LD et al (2021) Advances in non-equilibrium CO2 plasma kinetics: a theoretical and experimental review. Eur Phys J D 75(9):1–55
Wanten B et al (2023) Plasma-based CO2 conversion: How to correctly analyze the performance? J Energy Chem. https://doi.org/10.1016/j.jechem.2023.07.005
Peeters F, Van de Sanden M (2014) The influence of partial surface discharging on the electrical characterization of DBDs. Plasma Sources Sci Technol 24(1):015016
Jahanbakhsh S, Brüser V, Brandenburg R (2020) Experimental investigation of single microdischarges in a barrier corona arrangement with a cathodic metal pin. Plasma Sources Sci Technol 29(1):015001
Butterworth T, Elder R, Allen R (2016) Effects of particle size on CO2 reduction and discharge characteristics in a packed bed plasma reactor. Chem Eng J 293:55–67
Choi JH, Han MH, Baik HK (2008) Experimental analysis of reduced electric field during time-variable pulsed dielectric barrier discharge. Jpn J Appl Phys 47(2R):1071
Wang Y et al (2022) Catalyst-free single-step plasma reforming of CH4 and CO2 to higher value oxygenates under ambient conditions. Chem Eng J 450:137860
Kogelschatz U (1988) Advanced ozone generation. Process technologies for water treatment. Springer, pp 87–118
Brandenburg R et al (2023) Plane parallel barrier discharges for carbon dioxide splitting: influence of discharge arrangement on carbon monoxide formation. Plasma 6(1):162–180
Ramakers M et al (2015) Effect of argon or helium on the CO2 conversion in a dielectric barrier discharge. Plasma Process Polym 12(8):755–763
Lindon MA, Scime EE (2014) CO2 dissociation using the Versatile atmospheric dielectric barrier discharge experiment (VADER). Front Phys 2:55
George A et al (2021) A review of non-thermal plasma technology: a novel solution for CO2 conversion and utilization. Renew Sustain Energy Rev 135:109702
Brehmer F et al (2014) CO and byproduct formation during CO2 reduction in dielectric barrier discharges. J Appl Phys 116(12):123303
Uytdenhouwen Y et al (2021) On the kinetics and equilibria of plasma-based dry reforming of methane. Chem Eng J 405:126630
Brandenburg RS, Schmidt M, Höft H, Pipa AV, Brüser V (2023) Plane parallel barrier discharges for carbon dioxide splitting: influence of discharge arrangement on carbon monoxide formation. Plasma 6:162–180
Belov I, Paulussen S, Bogaerts A (2016) Appearance of a conductive carbonaceous coating in a CO2 dielectric barrier discharge and its influence on the electrical properties and the conversion efficiency. Plasma Sources Sci Technol 25(1):015023
Uytdenhouwen Y et al (2019) How process parameters and packing materials tune chemical equilibrium and kinetics in plasma-based CO2 conversion. Chem Eng J 372:1253–1264
Mei DH et al (2016) Optimization of CO2 conversion in a cylindrical dielectric barrier discharge reactor using design of experiments. Plasma Process Polym 13(5):544–556
Stankov M et al (2022) Extended reaction kinetics model for non-thermal argon plasmas and its test against experimental data. Plasma Sources Sci Technol 31:125002
Stewig C et al (2020) Excitation and dissociation of CO2 heavily diluted in noble gas atmospheric pressure plasma. J Phys D-Appl Phys 53(12):125205
Brandenburg R, Sarani A (2017) About the development of single microdischarges in dielectric barrier discharges in CO2 and CO2/N-2 gas mixtures. Eur Phys J Special Top 226(13):2911–2922
Brehmer F et al (2015) Gas temperature in transient CO2 plasma measured by Raman scattering. J Phys D Appl Phys 48(15):155201
Batakliev T et al (2014) Ozone decomposition. Interdiscip Toxicol 7(2):47
Thimsen E (2021) Entropy production and chemical reactions in nonequilibrium plasma. AIChE J 67(9):e17291
Alcantara ML et al (2021) Thermodynamic analysis of carbon dioxide conversion reactions. Case studies: formic acid and acetic acid synthesis. Ind Eng Chem Res 60(25):9246–9258
Mousazadeh Borghei S, Brüser V, Kolb JF (2023) Polarity dependence of CO2 conversion in nanosecond pulsed large gap dielectric barrier discharges. Eur Phys J D 77(3):36
Acknowledgements
Many thanks to our colleague Michael Schmidt for providing comments and technical help.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Federal Ministry of Education and Research (BMBF) under grant 01DK20067.
Author information
Authors and Affiliations
Contributions
HM: set-up of experiment and the analytics preparation; performance of experiments and analysis; writing of first draft. VB: development of overall research strategy; outline of the manuscript; thermodynamic calculations; revision and editing of manuscript. MS: set-up of experiment and analytics preparation; revision of analysis; revision and editing of manuscript. RB: development of overall research strategy; outline of the manuscript; revision of analysis revision and final editing of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahdikia, H., Brüser, V., Schiorlin, M. et al. CO2 Dissociation in Barrier Corona Discharges: Effect of Elevated Pressures in CO2/Ar Mixtures. Plasma Chem Plasma Process 43, 2035–2063 (2023). https://doi.org/10.1007/s11090-023-10411-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10411-1