Abstract
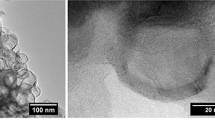
Boron nitride nanosheets (BNNS) were recently synthesized in a powder form using inductively coupled plasma through two readily-scalable bottom-up routes: (i) heterogeneous nucleation using amorphous boron particles and nitrogen, (ii) homogeneous nucleation from ammonia borane and nitrogen. The operating pressure was found to play a significant role in controlling the product purity and sheet dimensions in both routes. This work attempts to understand the effect of pressure by first presenting thermodynamic equilibrium calculations for the two systems at various pressures. From these, we estimate nucleation zones for BNNS and identify their possible major precursors. Computational fluid dynamics simulations (CFD) are then used to calculate plasma thermofluidic profiles by which axial residence times and gas cooling rates are estimated for the nucleation zones. Finally, in-situ optical emission spectroscopy (OES) is used to investigate the chemical composition of the gas during BNNS synthesis. It is found that the optimum pressure for the two routes is 62 kPa. The formation of BNNS heterogeneously follows a base-growth mechanism and requires the presence of liquid boron, B(liq) and N2/N/BN(g). The nucleation theory is used to explain the formation of BNNS homogeneously from BxNyHz critical clusters that grow into BNNS by the addition of BH/BN/NH onto the clusters. Thermodynamic equilibrium charts predict the formation of these species in their corresponding systems. Based on the species densities, BNNS formation/nucleation temperature ranges are proposed, e.g., around 2740–2350 K at the optimum pressure. The CFD simulation results at the formation/nucleation zones show that residence times and cooling rates control the formation of BNNS. These are found to be 12.4 ms and 34.1 × 103 K s−1, respectively, at the optimum operating pressure. OES spectra of both routes show the presence of several species consistent with the thermodynamic equilibrium results.










Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request from the authors.
References
Novoselov KS, Jiang D, Schedin F, Booth T, Khotkevich V, Morozov S, Geim AK (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci USA 102(30):10451–10453. https://doi.org/10.1073/pnas.0502848102
Cai Q, Scullion D, Gan W, Falin A, Zhang S, Watanabe K, Taniguchi T, Chen Y, Santos EJG, Li LH (2019) High thermal conductivity of high-quality monolayer boron nitride and its thermal expansion. Sci Adv 5(6):eaav0129. https://doi.org/10.1126/sciadv.aav0129
Meng W, Huang Y, Fu Y, Wang Z, Zhi C (2014) Polymer composites of boron nitride nanotubes and nanosheets. J Mater Chem C 2(47):10049–10061. https://doi.org/10.1039/C4TC01998A
Shuai C, Han Z, Feng P, Gao C, Xiao T, Peng S (2015) Akermanite scaffolds reinforced with boron nitride nanosheets in bone tissue engineering. J Mater Sci Mater Med 26(5):188. https://doi.org/10.1007/s10856-015-5513-4
Farshid B, Lalwani G, Mohammadi MS, Simonsen J, Sitharaman B (2017) Boron nitride nanotubes and nanoplatelets as reinforcing agents of polymeric matrices for bone tissue engineering. J Biomed Mater Res Part B Appl Biomater 105(2):406–419. https://doi.org/10.1002/jbm.b.33565
Falin A, Cai Q, Santos EJ, Scullion D, Qian D, Zhang R, Yang Z, Huang S, Watanabe K, Taniguchi T (2017) Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat Commun 8(1):1–9. https://doi.org/10.1038/ncomms15815
Guerra V, Wan C, Degirmenci V, Sloan J, Presvytis D, McNally T (2018) 2D boron nitride nanosheets (BNNS) prepared by high-pressure homogenisation: structure and morphology. Nanoscale 10(41):19469–19477. https://doi.org/10.1039/c8nr06429f
Golberg D, Bando Y, Huang Y, Terao T, Mitome M, Tang C, Zhi C (2010) Boron nitride nanotubes and nanosheets. ACS Nano 4(6):2979–2993. https://doi.org/10.1021/nn1006495
Sajjad M, Morell G, Feng P (2013) Advance in novel boron nitride nanosheets to nanoelectronic device applications. ACS Appl Mater Interfaces 5(11):5051–5056. https://doi.org/10.1021/am400871s
Sainsbury T, Satti A, May P, Wang Z, McGovern I, Gun’ko YK, Coleman J (2012) Oxygen radical functionalization of boron nitride nanosheets. J Am Chem Soc 134(45):18758–18771. https://doi.org/10.1021/ja3080665
Yu J, Qin L, Hao Y, Kuang S, Bai X, Chong Y-M, Zhang W, Wang E (2010) Vertically aligned boron nitride nanosheets: chemical vapor synthesis, ultraviolet light emission, and superhydrophobicity. ACS Nano 4(1):414–422. https://doi.org/10.1021/nn901204c
Pakdel A, Zhi C, Bando Y, Nakayama T, Golberg D (2011) Boron nitride nanosheet coatings with controllable water repellency. ACS Nano 5(8):6507–6515. https://doi.org/10.1021/nn201838w
Li LH, Chen Y, Cheng B-M, Lin M-Y, Chou S-L, Peng Y-C (2012) Photoluminescence of boron nitride nanosheets exfoliated by ball milling. Appl Phys Lett 100(26):261108. https://doi.org/10.1063/1.4731203
Lin L, Xu Y, Zhang S, Ross IM, Ong AC, Allwood DA (2014) Fabrication and luminescence of monolayered boron nitride quantum dots. Small 10(1):60–65. https://doi.org/10.1002/smll.201301001
Weng Q, Wang B, Wang X, Hanagata N, Li X, Liu D, Wang X, Jiang X, Bando Y, Golberg D (2014) Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 8(6):6123–6130. https://doi.org/10.1021/nn5014808
Anota EC, Tlapale Y, Villanueva MS, Márquez JAR (2015) Non-covalent functionalization of hexagonal boron nitride nanosheets with guanine. J Mol Model 21(8):215. https://doi.org/10.1007/s00894-015-2768-0
Garcia AG, Neumann M, Amet FO, Williams JR, Watanabe K, Taniguchi T, Goldhaber-Gordon D (2012) Effective cleaning of hexagonal boron nitride for graphene devices. Nano Lett 12(9):4449–4454. https://doi.org/10.1021/nl3011726
Lei W, Portehault D, Liu D, Qin S, Chen Y (2013) Porous boron nitride nanosheets for effective water cleaning. Nat Commun 4:1777. https://doi.org/10.1038/ncomms2818
Weng Q, Wang X, Bando Y, Golberg D (2014) One-Step Template-Free Synthesis of Highly Porous Boron Nitride Microsponges for Hydrogen Storage. Adv Energy Mater 4(7):1301525. https://doi.org/10.1002/aenm.201301525
Weng Q, Wang X, Zhi C, Bando Y, Golberg D (2013) Boron nitride porous microbelts for hydrogen storage. ACS Nano 7(2):1558–1565. https://doi.org/10.1021/nn305320v
Rasul MG, Kiziltas A, Arfaei B, Shahbazian-Yassar R (2021) 2D boron nitride nanosheets for polymer composite materials. npj 2D Mater Appl 5(1):1–18. https://doi.org/10.1038/s41699-021-00231-2
Chatterjee S, Luo Z, Acerce M, Yates DM, Johnson ATC, Sneddon LG (2011) Chemical vapor deposition of boron nitride nanosheets on metallic substrates via decaborane/ammonia reactions. Chem Mater 23(20):4414–4416. https://doi.org/10.1021/cm201955v
Lee KH, Shin H-J, Lee J, Lee I-Y, Kim G-H, Choi J-Y, Kim S-W (2012) Large-scale synthesis of high-quality hexagonal boron nitride nanosheets for large-area graphene electronics. Nano Lett 12(2):714–718. https://doi.org/10.1021/nl203635v
Shi Y, Hamsen C, Jia X, Kim KK, Reina A, Hofmann M, Hsu AL, Zhang K, Li H, Juang Z-Y (2010) Synthesis of few-layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett 10(10):4134–4139. https://doi.org/10.1021/nl1023707
Gibb AL, Alem N, Chen J-H, Erickson KJ, Ciston J, Gautam A, Linck M, Zettl A (2013) Atomic resolution imaging of grain boundary defects in monolayer chemical vapor deposition-grown hexagonal boron nitride. J Am Chem Soc 135(18):6758–6761. https://doi.org/10.1021/ja400637n
Glavin NR, Jespersen ML, Check MH, Hu J, Hilton AM, Fisher TS, Voevodin AA (2014) Synthesis of few-layer, large area hexagonal-boron nitride by pulsed laser deposition. Thin Solid Films 572:245–250. https://doi.org/10.1016/j.tsf.2014.07.059
Merenkov I, Kosinova M, Ermakova E, Maksimovskii E, Rumyantsev YM (2015) PECVD synthesis of hexagonal boron nitride nanowalls from a borazine + ammonia mixture. Inorg Mater 51(11):1097–1103. https://doi.org/10.1134/s0020168515100118
Li LH, Chen Y, Behan G, Zhang H, Petravic M, Glushenkov AM (2011) Large-scale mechanical peeling of boron nitride nanosheets by low-energy ball milling. J Mater Chem 21(32):11862–11866. https://doi.org/10.1039/c1jm11192b
Lee D, Lee B, Park KH, Ryu HJ, Jeon S, Hong SH (2015) Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling. Nano Lett 15(2):1238–1244. https://doi.org/10.1021/nl504397h
Li LH, Glushenkov AM, Hait SK, Hodgson P, Chen Y (2014) High-efficient production of boron nitride nanosheets via an optimized ball milling process for lubrication in oil. Sci Rep 4:07288. https://doi.org/10.1038/srep07288
Zhi C, Bando Y, Tang C, Kuwahara H, Golberg D (2009) Large-scale fabrication of boron nitride nanosheets and their utilization in polymeric composites with improved thermal and mechanical properties. Adv Mater 21(28):2889–2893. https://doi.org/10.1002/adma.200900323
Wang Y, Shi Z, Yin J (2011) Boron nitride nanosheets: large-scale exfoliation in methanesulfonic acid and their composites with polybenzimidazole. J Mater Chem 21(30):11371–11377. https://doi.org/10.1039/c1jm10342c
Khan U, May P, O’Neill A, Bell AP, Boussac E, Martin A, Semple J, Coleman JN (2013) Polymer reinforcement using liquid-exfoliated boron nitride nanosheets. Nanoscale 5(2):581–587. https://doi.org/10.1039/c2nr33049k
Li L, Li LH, Chen Y, Dai XJ, Lamb PR, Cheng BM, Lin MY, Liu X (2013) High-quality boron nitride nanoribbons: unzipping during nanotube synthesis. Angew Chem Int Ed 52(15):4212–4216. https://doi.org/10.1002/anie.201209597
Liao Y, Chen Z, Connell JW, Fay CC, Park C, Kim JW, Lin Y (2014) Chemical sharpening, shortening, and unzipping of boron nitride nanotubes. Adv Funct Mater 24(28):4497–4506. https://doi.org/10.1002/adfm.201400599
Wu P, Zhu W, Chao Y, Zhang J, Zhang P, Zhu H, Li C, Chen Z, Li H, Dai S (2016) A template-free solvent-mediated synthesis of high surface area boron nitride nanosheets for aerobic oxidative desulfurization. Chem Commun 52(1):144–147. https://doi.org/10.1039/c5cc07830j
Wang X, Pakdel A, Zhi C, Watanabe K, Sekiguchi T, Golberg D, Bando Y (2012) High-yield boron nitride nanosheets from ‘chemical blowing’: towards practical applications in polymer composites. J Phys 24(31):314205. https://doi.org/10.1088/0953-8984/24/31/314205
Yurdakul H, Göncü Y, Durukan O, Akay A, Seyhan AT, Ay N, Turan S (2012) Nanoscopic characterization of two-dimensional (2D) boron nitride nanosheets (BNNSs) produced by microfluidization. Ceram Int 38(3):2187–2193. https://doi.org/10.1016/j.ceramint.2011.10.064
Stagi L, Ren J, Innocenzi P (2019) From 2-D to 0-D boron nitride materials, the next challenge. Materials 12(23):3905. https://doi.org/10.3390/ma12233905
Ren J, Stagi L, Innocenzi P (2020) Hydroxylated boron nitride materials: from structures to functional applications. J Mater Sci 56:4053–4079. https://doi.org/10.1007/s10853-020-05513-6
Bernard S, Salameh C, Moussa G, Demirci UB, Miele P, Nanoceramics BN (2015). In: Kobayashi S, Müllen K (eds) Encyclopedia of polymeric nanomaterials. Springer, Berlin, pp 1–12
Khan MH, Liu HK, Sun X, Yamauchi Y, Bando Y, Golberg D, Huang Z (2017) Few-atomic-layered hexagonal boron nitride: CVD growth, characterization, and applications. Mater Today 20(10):611–628. https://doi.org/10.1016/j.mattod.2017.04.027
Jiang X-F, Weng Q, Wang X-B, Li X, Zhang J, Golberg D, Bando Y (2015) Recent progress on fabrications and applications of boron nitride nanomaterials: a review. J Mater Sci Technol 31(6):589–598. https://doi.org/10.1016/j.jmst.2014.12.008
Ferrari AG-M, Rowley-Neale SJ, Banks CE (2020) Recent advances in 2D hexagonal boron nitride (2D-hBN) applied as the basis of electrochemical sensing platforms. Anal Bioanal Chem 413(3):663–672. https://doi.org/10.1007/s00216-020-03068-8
Luo W, Wang Y, Hitz E, Lin Y, Yang B, Hu L (2017) Solution processed boron nitride nanosheets: synthesis, assemblies and emerging applications. Adv Funct Mater 27(31):1701450. https://doi.org/10.1002/adfm.201701450
Alrebh A, Meunier J-L (2021) Synthesis of boron nitride nanosheets powders using a plasma based bottom-up approach. 2D Mater 8(4):1045018. https://doi.org/10.1088/2053-1583/ac1854
Kumashiro Y, Yokoyama T, Sato A, Ando Y (1997) Thermoelectric properties of boron and boron phosphide CVD wafers. J Solid State Chem 133:314–321. https://doi.org/10.1109/ict.1998.740448
Mostaghimi J, Boulos MI (1989) Two-dimensional electromagnetic field effects in induction plasma modelling. Plasma Chem Plasma Process 9(1):25–44. https://doi.org/10.1007/bf01015825
Boulos MI (1985) The inductively coupled RF (radio frequency) plasma. Pure Appl Chem 57(9):1321–1352. https://doi.org/10.1351/pac198557091321
Pristavita R, Mendoza-Gonzalez N-Y, Meunier J-L, Berk D (2010) Carbon blacks produced by thermal plasma: the influence of the reactor geometry on the product morphology. Plasma Chem Plasma Process 30(2):267–279. https://doi.org/10.1007/s11090-010-9218-7
Pristavita R, Mendoza-Gonzalez N-Y, Meunier J-L, Berk D (2011) Carbon nanoparticle production by inductively coupled thermal plasmas: controlling the thermal history of particle nucleation. Plasma Chem Plasma Process 31(6):851–866. https://doi.org/10.1007/s11090-011-9319-y
Meunier J-L, Mendoza-Gonzalez NY, Pristavita R, Binny D, Berk D (2013) Homogeneous nucleation of graphene nanoflakes (GNFs) in thermal plasma: tuning the 2D nanoscale geometry. 21st international symposium on plasma chemistry
Legrand U, Gonzalez N-YM, Pascone P, Meunier J-L, Berk D (2016) Synthesis and in-situ oxygen functionalization of deposited graphene nanoflakes for nanofluid generation. Carbon 102:216–223. https://doi.org/10.1016/j.carbon.2016.02.043
Meunier J-L, Mendoza-Gonzalez N-Y, Pristavita R, Binny D, Berk D (2014) Two-dimensional geometry control of graphene nanoflakes produced by thermal plasma for catalyst applications. Plasma Chem Plasma Process 34(3):505–521. https://doi.org/10.1007/s11090-014-9524-6
Kim KS, Hong SH, Lee K-S, Ju WT (2007) Continuous synthesis of nanostructured sheetlike carbons by thermal plasma decomposition of methane. IEEE T Plasma Sci 35(2):434–443. https://doi.org/10.1109/tps.2007.892556
Gonzalez NYM, El Morsli M, Proulx P (2008) Production of nanoparticles in thermal plasmas: a model including evaporation, nucleation, condensation, and fractal aggregation. J Therm Spray Technol 17(4):533–550. https://doi.org/10.1007/s11666-008-9209-x
Boulos MI (1991) Thermal Plasma Processing. IEEE T Plasma Sci 19(6):1078–1089. https://doi.org/10.1109/27.125032
Boulos MI, Fauchais P, Pfender E (1994) Thermal plasmas: fundamentals and applications. Springer Science & Business Media, Berlin
Castillo IA, Munz R (2007) New in-situ sampling and analysis of the production of CeO2 powders from liquid precursors using a novel wet collection system in a rf inductively coupled thermal plasma reactor part. 1: reactor system and sampling probe. Plasma Chem Plasma Process 27(6):737–759. https://doi.org/10.1007/s11090-007-9105-z
Kim K, Moradian A, Mostaghimi J, Soucy G (2010) Modeling of induction plasma process for fullerene synthesis: effect of plasma gas composition and operating pressure. Plasma Chem Plasma Process 30(1):91–110. https://doi.org/10.1007/s11090-009-9211-1
Kim KS, Couillard M, Shin H, Plunkett M, Ruth D, Kingston CT, Simard B (2018) Role of hydrogen in high-yield growth of boron nitride nanotubes at atmospheric pressure by induction thermal plasma. ACS Nano 12(1):884–893. https://doi.org/10.1021/acsnano.7b08708
Mostaghimi J, Proulx P, Boulos MI (1985) An analysis of the computer modeling of the flow and temperature fields in an inductively coupled plasma. Numer Heat Trans 8(2):187–201. https://doi.org/10.1080/01495728508961849
Santra B, Ko H-Y, Yeh Y-W, Martelli F, Kaganovich I, Raitses Y, Car R (2018) Root-growth of boron nitride nanotubes: experiments and ab initio simulations. Nanoscale 10(47):22223–22230. https://doi.org/10.1039/c8nr06217j
Khrabry A, Kaganovich ID, Yatom S, Vekselman V, Radić-Perić J, Rodman J, Raitses Y (2019) Determining the gas composition for the growth of BNNTs using a thermodynamic approach. Phys Chem Chem Phys 21(24):13268–13286. https://doi.org/10.1039/c9cp01342c
Sugiyama K, Akazawa K, Oshima M, Miura H, Matsuda T, Nomura O (1986) Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem Plasma Process 6(2):179–193. https://doi.org/10.1007/bf00571275
Mohajeri N, Ali T, Ramasamy KK (2007) Thermal conductivity of ammonia borane complex and its composites with aluminum powder. Thermochim Acta 452(1):28–30. https://doi.org/10.1016/j.tca.2006.10.012
Krstic PS, Han L, Irle S, Nakai H (2018) Simulations of the synthesis of boron-nitride nanostructures in a hot, high pressure gas volume. Chem Sci 9(15):3803–3819. https://doi.org/10.1039/c8sc00667a
Kim KS, Kingston CT, Hrdina A, Jakubinek MB, Guan J, Plunkett M, Simard B (2014) Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 8(6):6211–6220. https://doi.org/10.1021/nn501661p
NIST ASD Team (2021) NIST Atomic Spectra Database Lines. National Institute of Standards and Technology, NIST. https://www.nist.gov/pml/atomic-spectra-database. Accessed Dec 2021
Pearse RWB, Gaydon AG, Pearse RWB, Gaydon AG (1976) The identification of molecular spectra, 4th edition. Chapman and Hall, New York
Pierson J, Czerwiec T, Belmonte T, Michel H, Ricard A (1998) Kinetics of boron atoms in Ar-BCl3 flowing microwave discharges. Plasma Sources Sci Technol 7(1):54. https://doi.org/10.1088/0963-0252/7/1/008
Savkin KP, Bugaev AS, Gushenets VI, Nikolaev AG, Ivanov YF, Oks EM, Frolova VP, Shandrikov MV, Yushkov GY (2020) Generation of micron and submicron particles in atmospheric pressure discharge in argon flow with magnesium, zinc, and boron carbide electrodes. Surf Coat Technol 389:125578. https://doi.org/10.1016/j.surfcoat.2020.125578
Hrycak B, Jasiński M, Mizeraczyk J (2012) Spectroscopic characterization of nitrogen plasma generated by waveguide-supplied coaxial-line-based nozzleless microwave source. J Phys: Conf Ser 406(1):012037. https://doi.org/10.1088/1742-6596/406/1/012037
Mavadat M, Ricard A, Sarra-Bournet C, Laroche G (2011) Determination of ro-vibrational excitations of N2 (B, v′) and N2 (C, v′) states in N2 microwave discharges using visible and IR spectroscopy. J Phys D 44(15):155207. https://doi.org/10.1088/0022-3727/44/15/155207
Yatom S, Raitses Y (2020) Characterization of plasma and gas-phase chemistry during boron-nitride nanomaterial synthesis by laser-ablation of boron-rich targets. Phys Chem Chem Phys 22(36):20837–20850. https://doi.org/10.1039/D0CP02890H
Glavin NR, Muratore C, Jespersen ML, Hu J, Fisher TS, Voevodin AA (2015) Temporally and spatially resolved plasma spectroscopy in pulsed laser deposition of ultra-thin boron nitride films. J Appl Phys 117(16):165305. https://doi.org/10.1063/1.4919068
Barni R, Alex P, Ghorbanpour E, Riccardi C (2021) A spectroscopical study of H2 emission in a simply magnetized toroidal plasma. The Eur Phys J D 75(3):1–6. https://doi.org/10.1140/epjd/s10053-021-00109-4
Song MA, Lee YW, Chung TH (2011) Characterization of an inductively coupled nitrogen-argon plasma by Langmuir probe combined with optical emission spectroscopy. Phys Plasmas 18(2):023504. https://doi.org/10.1063/1.3554706
Czerwiec T, Gavillet J, Belmonte T, Michel H, Ricard A (1998) Determination of O atom density in Ar-O2 and Ar-O2-H2 flowing microwave discharges. Surf Coat Technol 98(1–3):1411–1415. https://doi.org/10.1016/s0257-8972(97)00256-9
Czerwiec T, Greer F, Graves D (2005) Nitrogen dissociation in a low pressure cylindrical ICP discharge studied by actinometry and mass spectrometry. J Phys D 38(24):4278. https://doi.org/10.1088/0022-3727/38/24/003
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). We, the authors, acknowledge with deep appreciation the contribution of our former colleague and lab member Dr. Norma-Yadira Mendoza-Gonzalez for her meticulous training and the continuous support on Ansys-FLUENT through which the results on CFD modeling were acquired.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alrebh, A., Meunier, JL. Boron Nitride Nanosheets Synthesis in Thermal Plasma: An Experimental and Modelling Analysis. Plasma Chem Plasma Process 42, 855–884 (2022). https://doi.org/10.1007/s11090-022-10245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10245-3