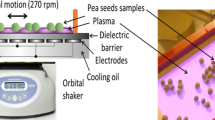
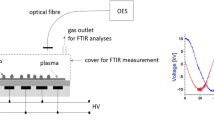
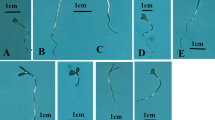
Abstract
Improving the germination of economically important crops and the condition of young plants is a major challenge currently facing agricultural practice. Pea (Pisum sativum L.) is one of the four most important cultivated legumes, along with groundnut (Arachis hypogaea L.), soybean (Glycine max L.) and beans (Phaseolus vulgaris L.). Due to the high protein content (23–33%), there is an interest in growing this crop as a source of protein for humans and animals. In this study, we focused on the effect of Cold Atmospheric Pressure Plasma (CAPP) on the decontamination and germination of pea seeds, on young seedling growth and production parameters, and on increasing their resistance and mechanical strength. We can state that germination increased by 10 to 25% after plasma treatment, and the most significant decontamination effect was detected when using non-thermal plasma generated in the ambient air (A-variants) and in the nitrogen atmosphere (N-variants). The increased in situ activity of peroxidases (POX) in the cell walls of A-variants and N-variants is also closely related to the increase in the mechanical strength of the cell walls and thus contributes to the higher resistance of these seedlings. This is also illustrated by the differences in lignin deposition among the different variants after CAPP treatment. To our knowledge, this is the first study concerning the influence of CAPP on the lignification of root tissues and on increasing the strength and resistance of plants.











Similar content being viewed by others
References
Chen FF (2016) Plasma applications. Springer, Switzerland, Introduction to plasma physics and controlled fusion. https://doi.org/10.1007/978-3-319-22309-4_10
Šimončicová J, Kryštofová S, Medvecká V, Ďurišová K, Kaliňáková B (2019) Technical applications of plasma treatments: current state and perspectives. Appl Microbiol Biotechnol [online] 103:5117–5129
Hertwig C, Meneses N, Mathys A (2018) Cold atmospheric pressure plasma and low energy electron beam as alternative nonthermal decontamination technologies for dry food surfaces: a review. Trends Food Sci Technol [online] 77:131–142
Moreau M, Orange N, Feuilloley MGJ (2008) Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv 26:610–617
Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233:81–86
Larroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the art. Plasma Process Polym 2:391–400
Bourke P, Ziuzina D, Boehm D, Cullen PJ, Keener K (2018) The potential of cold plasma for safe and sustainable food production. Trends Biotechnol 36:615–626
Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H, Hanliang S, Yuanhua D (2014) Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci Rep 4:5859
Meng Y, Qu G, Wang T, Sun Q, Liang D, Hu S (2017) Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem Plasma Process 37:1105–1119
Kyzek S, Holubová Ľ, Medvecká V, Tomeková J, Gálová E, Zahoranová A (2019) Cold atmospheric pressure plasma can induce adaptive response in pea seeds. Plasma Chem Plasma Process 39:475–486
Stolárik T, Henselová M, Martinka M, Novák O, Zahoranová A, Černák M (2015) Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem Plasma Proces 35:659–676
Ji SH, Choi KH, Pengkit A, Im JS, Kim JS, Kim YH, Park Y, Hong EJ, Jung SK, Choi EH, Park G (2016) Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch Biochem Biophys 605:117–128
Bormashenko E, Grynyov R, Bormashenko Y, Drori E (2012) Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci Rep 2:3–10
Bormashenko B, Shapira Y, Grynyov R, Whyman G, Bormashenko Y, Drori E (2015) Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J Exp Bot 66(13):4013–4021
Nishime TMC, Wannicke N, Horn S, Weltmann KD, Brust H (2020) A coaxial dielectric barrier discharge reactor for treatment of winter wheat seeds. Appl Sci 10:7133. https://doi.org/10.3390/app10207133
Dobrin D, Magureanu M, Mandache NB, Ionita MD (2015) The effect of non-thermal plasma treatment on wheat germination and early growth. Innov Food Sci Emerg Technol 29:255–260
Sera B, Sery M, Gavril B, Gajdova I (2017) Seed germination and early growth responses to seed pre-treatment by non-thermalplasma in hemp vultivars (Cannabis sativa L.). Plasma Chem Plasma Process 37:207–221
Zahoranová A, Henselová M, Hudecová D, Kaliňáková B, Kováčik D, Medvecká V, Černák M (2016) Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem Plasma Process 36:397–414
Panngom K, Lee SH, Park DH, Sim GB, Kim YH, Uhm HS, Park G, Choi EH (2014) Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS ONE 9:e99300
Kordas L, Pusz W, Czapka T, Kacprzyk R (2015) The effect of low-temperature plasma on fungus colonization of winter wheat grain and seed quality. Polish J Environ Stud 24:433–438
Zahoranová A, Hoppanová L, Šimončicová J, Tučeková Z, Medvecká M, Hudecová D, Kaliňáková B, Kováčik D, Černák M (2018) Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem Plasma Proces 38:969–988
Tensmeyer LG, Wright PE, Fegenbush DO, Snapp SW (1981) Sterilization of glass containers by laser initiated plasmas. J Parenter Sci Technol 35:93–97
Nelson CL, Berger TJ (1989) Inactivation of microorganisms by oxygen gas plasma. Curr Microbiol 18:275–276
Švubová R, Kyzek S, Medvecká V, Slováková Ľ, Gálová E, Zahoranová A (2020) Novel insight at the effec of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. cv. Prophet) seeds. Plasma Chem Plasma Proces 40:1221–1240
Bafoil M, Jemmat A, Martinez Y, Merbahi N, Eichwald O, Dunand C, Yousfi M (2018) Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 13:e0195512
Yemeli GBN, Švubová R, Kostoláni D, Kyzek S, Machala Z (2020) The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process Polym 18(1):2000205
Jiang J, Lu Y, Li J, Li L, He X, Shao H, Dong Y (2014) Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). PLoS ONE 9:e97753
Černák M, Černáková L, Hudec I, Černák M, Černáková L, Hudec I (2009) Difuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur Phys J Appl Phys 47:22806
Tomeková J, Kyzek S, Medvecká V, Gálová E, Zahoranová A (2020) Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem Plasma Proces 40:1571–1584
Abdul-Baki A, Anderson J (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Matušíková I, Salaj J, Moravčíková J, Mlynárová L, Nap J-P, Libantová J (2005) Tentacles of in vitro-grown round-leaf sundew Drosera rotundifolia L. show induction of chitinase activity upon mimicking the presence of prey. Planta 222(6):1020–1027
Kumar D, Yusuf M, Singh P, Sardar M, Sarin NB (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc 4:3–6
Frič F, Fuchs W (1970) Veränderungen der Aktivität einiger Enzyme im Weizenblatt in Abhängigkeit von der temperaturlabilen Verträglichkeit für Puccinia graminis tritici. J Phytopathol 67:161–174
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Ferrer MA, Calderón AA, Muñoz R, Ros Barceló A (1990) 4-methoxy-a-naphthol as a specific substrate for kinetic, zymographic and cytochemical studies on plant peroxidase activities. Phytochem Anal 1:63–69
Ďurčeková K, Huttová J, Mistrík I, Olle M, Tamás L (2007) Cadmium induces premature xylogenesis in barley roots. Plant Soil 290:61–68
Gichner T, Patková Z, Száková J, Žnidar I, Mukherjee A (2008) DNA damage in potato plants induced by cadmium, ethyl methanesulphonate and γ-rays. Environ Exp Bot 62:113–119
Dhayal M, Lee SY, Park SU (2006) Using low-pressure plasma for Carthamus tinctorium L seed surface modification. Vacuum 80(5):499–506
Švubová R, Slováková Ľ, Holubová Ľ, Rovňanová D, Gálová R, Tomeková J (2021) Evaluation of the impact of cold atmospheric pressure plasma on soybean seed germination. Plants Basel 10:177. https://doi.org/10.3390/plants10010177
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (eds) (2013) Seeds Physiology of development, germination and dormancy. Springer, New York
Švubová R, Kročková V, Renčko J, Slováková Ľ, Zahoranová A (2018). Vplyv nízkoteplotnej plazmy na klíčenie hospodársky významných plodín (Effect of low-temperature plasma on germination of agriculturally important crops). In: Vliv abiotických a biotických stresorů na vlastnosti rostlin 2018 - Zvolen, 05.09.2018 - 06.09.2018. SK
Renčko J, Medvecká V, Slováková Ľ, Zahoranová A (2019). Účinok nízkoteplotnej plazmy na procesy klíčenia jarného jačmeňa (Hordeum vulgare L. cv. Malz). (Impact of low-temperature plasma on germination process of spring barley (Hordeum vulgare L. cv. Malz). In: Študentská vedecká konferencia PriF UK 2019 - Bratislava, 09.04.2019. SK
Kročková V, Medvecká V, Švubová R, Zahoranová A (2019). Vplyv nízkoteplotnej plazmy (NTP) na klíčenie a antioxidačnú odozvu mladých klíčencov kukurice siatej (Zea mays L. hybrid Ronaldinio). (The influence of low-temperature plasma (NTP) on germination and antioxidative response of yong Zea mays L. (hybrid Ronaldinio) seedlings). In: Študentská vedecká konferencia PriF UK 2019 - Bratislava, 09.04.2019. SK
Di Pietro AD, Madrid MP, Caracuel Z, Delgado-Jarana J, Roncero MIG (2003) Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol Plant Pathol 4(5):315–325
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502
Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia L’H, (2001) Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 226:1–21
Zheng J, Su Ch, Zhou J, Xu L, Qian Y, Chen H (2017) Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem Eng J 317:309–316
Kang MH, Pengkit A, Choi K, Jeon SS, Choi HW, Shin DB, Choi EH, Uhm HS, Park G (2015) Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE. https://doi.org/10.1371/journal.pone.0139263
Khamsen N, Onwimol D, Teerakawanich N, Dechanupaprittha S, Kanokbannakorn W, Hongesombut K, Srisonphan S (2016) Rice Oryza sativa L seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.6b04555
Tadege M, Dupuis I, Kuhlemeier C (1999) Ethanolic fermentation: new functions for an old pathway. Trends Plant Sci 4:320–324
Su L, Lan Q, Pritchard HW, Xue H, Wang X (2016) Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol Bioch 109:406–415
Mildažienė V, Aleknavičiūtė V, Žūkienė R, Paužaitė G, Naučienė Z, Filatova I, Lyushkevich V, Haimi P, Tamošiūnė I, Baniulis D (2019) Treatment of common sunflower Helianthus annus L seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci Rep 9(1):1–12
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00800
Henselová M, Slováková Ľ, Martinka M, Zahoranová A (2012) Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 67:490–497
Kučerová K, Henselová M, Slováková Ľ, Hensel K (2019) Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process Polym 16:e1800131
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF (2012) Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep 39(2):969–987
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13:171–201
Begović L, Lepeduš H, Lalić A, Štolfa I, Jurković Z, Kovačević J, Cesar V (2017) Involvement of peroxidases in structural changes of barley stem. Bragantia [online] 76:52–359
Vaculik M, Landberg T, Greger M, Luxova M, Stolarikova M, Lux A (2012) Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann Bot 110(2):433–443
Vatehova Z, Kollarova K, Zelko I, Richterova-Kucerova D, Bujdos M, Liskova D (2012) Interaction of silicon and cadmium in Brassica juncea and Brassica napus. Biologia 67:498–504
Lukacova Z, Svubova R, Kohanova J, Lux A (2013) Silicon mitigates the Cd toxicity in maize in relation to cadmium translocation, cell distribution, antioxidant enzymes stimulation and enhanced endodermal apoplasmic barrier development. Plant Growth Regul 70:89–103
Masarovič D, Slováková Ľ, Bokor B, Bujdoš M, Lux A (2012) Effect of silicon application on Sorghum bicolor exposed to toxic concentration of zinc. Biologia 67:706–712
Lux A, Luxová M, Hattori T, Inanaga S, Sugimoto Y (2002) Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol Plant 115(1):87–92
Lukacova Z, Švubová R, Janikovicova S, Volajova Z, Lux A (2019) Tobacco plants (Nicotiana benthamiana) were influenced by silicon and were not infected by dodder (Cuscuta europaea). Plant Physiol Biochem 139:179–190
Acknowledgement
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-16-0216 and VEGA No. 1/0410/18. Authors would like to express thanks doc. RNDr. Anna Zahoranová, PhD. for help and many valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Renáta, Š., Nicolette, V., Monika, B. et al. Enhanced In situ Activity of Peroxidases and Lignification of Root Tissues after Exposure to Non-Thermal Plasma Increases the Resistance of Pea Seedlings. Plasma Chem Plasma Process 41, 903–922 (2021). https://doi.org/10.1007/s11090-021-10160-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10160-z