Abstract
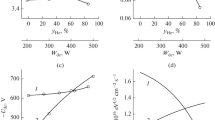
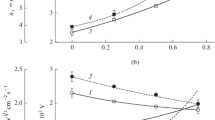
In this work, we investigated the possibidlxlity to control both gas-phase chemistry and silicon etching kinetics in C4F8 + O2 + Ar inductively coupled plasma by changes in O2/Ar, C4F8/O2 and C4F8/Ar mixing ratios at the constant fraction of the rest component (50%), gas pressure (10 mTorr), input power (700 W) and bias power (200 W). The combination of plasma diagnostics and modeling tools allowed one: (a) to compare the effects of gas mixing ratios on both steady-state plasma parameters and densities of active species; (b) to figure out key processes which determine the fluorine atom formation/decay balance in each gas system; and (c) to analyze the differences in Si etching kinetics in terms of process-condition-dependent effective reaction probability. It was shown that the maximum changes in gas-phase chemistry take place in O2-rich plasmas due to CFx + O/O(1D) → CFx−1O + F, CFxO + e → CFx−1O + F + e and CFO + O/O(1D) → CO2 + F stepwise dissociation pathways. It was suggested also that the effective probability for Si + xF → SiFx reaction may be controlled by either fluorocarbon film thickness (in C4F8—rich plasmas) or O atom flux (in Ar and O2—rich plasmas) through the balance of adsorption sites on the etched surface.




Similar content being viewed by others
References
Sugano T (1985) Applications of plasma processes to VLSI technology. Wiley, New York
Tauber RN, Wolf S (2000) Silicon processing for the VLSI erall. Process Technology, vol 1. Lattice Press, New York
Roth JR (1995) Industrial plasma engineering. Institute of Physics Publishing, Philadelphia
Adams AC, Sze SM (1988) VLSI technology. McGraw-Hill, New York
Lieberman MA, Lichtenberg AJ (2005) Principles of plasma discharges and materials processing. Wiley, New York
Rauf S, Ventzek PL (2002) Model for an inductively coupled Ar/c-C4F8 plasma discharge. J Vac Sci Technol A 20(1):14–23
Kokkoris G, Goodyear A, Cooke M, Gogolides E (2008) A global model for C4F8 plasmas coupling gas phase and wall surface reaction kinetics. J Phys D Appl Phys 41(19):195211
Vasenkov AV, Li X, Oehrlein GS, Kushner MJ (2004) Properties of c-C4F8 inductively coupled plasmas. II. Plasma chemistry and reaction mechanism for modeling of Ar/c-C4F8/O2 discharges. J Vac Sci Technol A 22(3):511–530
Zhao SX, Zhang YR, Gao F, Wang YN, Bogaerts A (2015) Bulk plasma fragmentation in a C4F8 inductively coupled plasma: a hybrid modeling study. J Appl Phys 117(24):243303
Kazumi H, Hamasaki R, Tago K (1996) Model prediction of radical composition in plasmas and correlation with measured etch characteristics of silicon dioxide. Plasma Sources Sci Technol 5(2):200
Li X, Ling L, Hua X et al (2003) Effects of Ar and O2 additives on SiO2 etching in C4F8-based plasmas. J Vac Sci Technol A 21(1):284–293
Li X, Ling L, Hua X et al (2003) Characteristics of C4F8 plasmas with Ar, Ne, and He additives for SiO2 etching in an inductively coupled plasma (ICP) reactor. J Vac Sci Technol A 21(6):1955–1963
Matsui M, Tatsumi T, Sekine M (2001) Relationship of etch reaction and reactive species flux in C4F8/Ar/O2 plasma for SiO2 selective etching over Si and Si3N4. J Vac Sci Technol A 19(5):2089–2096
Sankaran A, Kushner MJ (2005) Etching of porous and solid SiO2in Ar/c-C4F8, O2/c-C4F8 and Ar/O2/c-C4F8 plasmas. J Appl Phys 97(2):023307
Standaert TEFM, Hedlund C, Joseph EA et al (2004) Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J Vac Sci Technol A 22(1):53–60
Chun I, Efremov A, Yeom GY, Kwon KH (2015) A comparative study of CF4/O2/Ar and C4F8/O2/Ar plasmas for dry etching applications. Thin Solid Films 579:136–143
Lee J, Efremov A, Yeom GY et al (2015) Application of Si and SiO2 etching mechanisms in CF4/C4F8/Ar inductively coupled plasmas for nanoscale patterns. J Nanosci Nanotechno 15(10):8340–8347
Lim N, Efremov A, Kwon KH (2019) Gas-phase chemistry and etching mechanism of SiNx thin films in C4F8 + Ar inductively coupled plasma. Thin Solid Films 685:97–107
Sasaki K, Kawai Y, Kadota K (1999) Determination of fluorine atom density in reactive plasmas by vacuum ultraviolet absorption spectroscopy at 95.85 nm. Rev Sci Instrum 70(1):76–81
Kimura T, Noto M (2006) Experimental study and global model of inductively coupled CF4∕ O2 discharges. J Appl Phys 100(6):063303
Plumb IC, Ryan KR (1986) A model of the chemical processes occurring in CF4/O2 discharges used in plasma etching. Plasma Chem Plasma Process 6(3):205–230
Efremov A, Lee J, Kim J (2017) On the control of plasma parameters and active species kinetics in CF4 + O2 + Ar gas mixture by CF4/O2 and O2/Ar mixing ratios. Plasma Chem Plasma Process 37(5):1445–1462
Shun’ko E (2008) Langmuir probe in theory and practice. Universal Publishers, Boca Raton
Kwon KH, Efremov A, Kim M et al (2010) A model-based analysis of plasma parameters and composition in HBr/X (X = Ar, He, N2) inductively coupled plasmas. J Electrochem Soc 157(5):H574–H579
Hsu CC, Nierode MA, Coburn JW, Graves DB (2006) Comparison of model and experiment for Ar, Ar/O2 and Ar/O2/Cl2 inductively coupled plasmas. J Phys D Appl Phys 39(15):3272
Lee BJ, Lee BJ, Efremov A et al (2016) Etching characteristics and mechanisms of MoS2 2D Crystals in O2/Ar inductively coupled plasma. J Nanosci Nanotechno 16(11):11201–11209
Bose D, Rauf S, Hash DB et al (2004) Monte Carlo sensitivity analysis of CF2 and CF radical densities in ac-C4F8 plasma. J Vac Sci Technol A 22(6):2290–2298
Font GI, Morgan WL, Mennenga G (2002) Cross-section set and chemistry model for the simulation of c-C4F8 plasma discharges. J Appl Phys 91(6):3530–3538
Gray DC, Tepermeister I, Sawin HH (1993) Phenomenological modeling of ion-enhanced surface kinetics in fluorine-based plasma etching. J Vac Sci Technol B 11(4):1243–1257
Winters HF, Coburn JW, Chuang TJ (1983) Surface processes in plasma-assisted etching environments. J Vac Sci Technol B 1(2):469–480
Coburn JW (1982) Plasma etching and reactive ion etching. AVS monograph series. AIP, New York
Stoffels WW, Stoffels E, Tachibana K (1998) Polymerization of fluorocarbons in reactive ion etching plasmas. J Vac Sci Technol A 16(1):87–95
Lee J, Kim J, Efremov A et al (2019) Etching mechanisms and surface conditions for SiOxNy thin films in CF4 + CHF3 + O2 inductively coupled plasma. Plasma Chem Plasma Process 39(4):1127–1144
Schaepkens M, Standaert TEFM, Rueger NR et al (1999) Study of the SiO2-to-Si3N4 etch selectivity mechanism in inductively coupled fluorocarbon plasmas and a comparison with the SiO2-to-Si mechanism. J Vac Sci Technol A 17(1):26–37
Cunge G, Kogelschatz M, Joubert O, Sadeghi N (2005) Plasma-wall interactions during silicon etching processes in high-density HBr/Cl2/O2 plasmas. Plasma Sources Sci Technol 14:S42–S52
Kim DK, Kim YK, Lee H (2007) A study of the role of HBr and oxygen on the etch selectivity and the post-etch profile in a polysilicon/oxide etch using HBr/O2 based high density plasma for advanced DRAMs. Mat Sci Semicon Proc 10(1):41–48
Acknowledgements
The work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20172010105910) (B. J. Lee, Y. Nam and K.-H. Kwon).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, B.J., Efremov, A., Nam, Y. et al. Plasma Parameters and Silicon Etching Kinetics in C4F8 + O2 + Ar Gas Mixture: Effect of Component Mixing Ratios. Plasma Chem Plasma Process 40, 1365–1380 (2020). https://doi.org/10.1007/s11090-020-10097-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10097-9