Abstract
In this work, we report on the atomic layer deposition (ALD) of HfNx thin films by employing CpHf(NMe2)3 as the Hf(IV) precursor and Ar–H2 plasma in combination with external RF substrate biasing as the co-reactant. Following up on our previous results based on an H2 plasma and external RF substrate biasing, here we address the effect of ions with a larger mass and higher energy impinging on HfNx film surface during growth. We show that an increase in the average ion energy up to 304 eV leads to a very low electrical resistivity of 4.1 × 10–4 Ωcm. This resistivity value is achieved for films as thin as ~ 35 nm, and it is an order of magnitude lower than the resistivity reported in literature for HfNx films grown by either CVD or ALD, while being comparable to the resistivity of PVD-grown HfNx films. From the extensive thin film characterization, we conclude that the impinging ions during the film growth lead to the very low electrical resistivity of HfNx films by suppressing the oxygen incorporation and in-grain nano-porosity in the films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conductive transition metal nitride (TMN) films find many applications in nano-electronics. They are used as metal electrodes in metal oxide semiconductor field effect transistors (MOSFETs) [1,2,3,4], and as diffusion barriers in inter-connects [5,6,7,8]. In view of the continuous scaling of semiconductor devices, the application of TMN films at small dimensions requires ultra-thin films with low resistivity, besides forming stable interfaces, e.g. with the underlying high-k HfO2 [1, 3, 4, 8]. Specifically, thin films of titanium nitride and tantalum nitride tend to form undesirable oxy-nitrides at the interface with HfO2 [4, 8]. Low resistivity hafnium nitride (HfNx) can serve as an effective alternative because of its superior stability when used in combination with HfO2 [8].
HfNx predominantly exists in two crystal phases: highly resistive Hf3N4 with Hf(IV) oxidation state [9, 10], and low resistivity δ-HfN with Hf(III) oxidation state [11, 12]. The control of the oxidation state of Hf is therefore essential to synthesize conductive HfNx layers [13,14,15]. Physical vapor deposition (PVD) methods have been widely adopted for the synthesis of low resistivity HfNx films [8, 11, 12, 16,17,18,19]. Seo et al. have reported the growth of stoichiometric and epitaxial 500 nm thick HfNx layers with a resistivity of 1.4 × 10–5 Ωcm, which is the lowest resistivity value reported thus far [18]. Typically, the polycrystalline HfNx films prepared by PVD exhibit a resistivity of ca. (1–2)·10–4 Ωcm for a typical film thickness of ~ 200 nm [17, 19, 20]. On the other hand, the growth of low resistivity HfNx films by techniques employing a metal–organic precursor, such as chemical vapor deposition (CVD) or atomic layer deposition (ALD), is very challenging [13, 14, 21,22,23]. A major challenge is the reduction of Hf(IV) oxidation state in the precursor to Hf(III) oxidation state in the deposited film, as highlighted in our previous work [13, 14]. Kim et al. reported the growth of HfNx films with a resistivity of 1 × 10–3 Ωcm, the lowest achieved by means of CVD [21].
The urgent requirement from the field of nano-electronics is the synthesis of ultra-thin films with precise control over film thickness, excellent uniformity and conformality on high aspect ratio 3D nanostructures [24,25,26,27]. These requirements motivate the synthesis of low resistivity HfNx films by ALD. We have recently shown that the δ-HfN phase can be achieved by adopting CpHf(NMe2)3 as Hf(IV) precursor and H2 plasma as reducing co-reactant in a plasma-assisted ALD process [13]. We demonstrated that the application of an external RF substrate bias during the H2 plasma exposure and an increase in the time-averaged substrate potential (|Vbias|) from 0 to 130 V resulted in a major decrease in electrical resistivity (ρe) from 0.9 to 3.3 × 10–3 Ωcm [14]. The decrease in ρe was found to be correlated with a major increase in the fraction of Hf(III) oxidation state from 0.65 ± 0.02 to 0.82 ± 0.02 [13, 14]. These results demonstrated that the impingement of energetic ions during the film growth can significantly improve the chemical and associated electrical properties of HfNx thin films prepared by ALD. In parallel, Villamayor et al. recently showed that an increase in the mass of impinging ions positively affects also the crystallinity of HfNx films grown by PVD, which contributes to the decrease in electrical resistivity [20].
In the present work, we investigate the effect of Ar–H2 plasma in combination with external RF substrate biasing during the plasma half cycle as the reducing co-reactant. The impact of impingement of ions with larger mass and higher energy on the chemical and microstructural properties of HfNx films is addressed here. The motivation to use Ar–H2 plasma derives from the work of Sode et al. [28]. ArH+ is anticipated to be the most abundant ion in an Ar–H2 plasma, in contrast to the lighter H3+ ion in a H2 plasma as employed in the previous work [28]. Furthermore, the ion energy measurements carried out in the present work indicate that the growing HfNx film is subjected to a higher average ion energy (< Eion >) in the case of Ar–H2 plasma with respect to the previously reported H2 plasma process [14]. The ions with a larger mass and higher < Eion > leads to a minimum in the electrical resistivity of 4.1 × 10–4 Ωcm. To best of our knowledge, this value represents the lowest resistivity reported in the literature for HfNx films grown by either CVD or ALD, and is comparable to the resistivity of PVD grown films [13, 14, 21,22,23]. This low resistivity is achieved for films as thin as ~ 35 nm. As a result of impinging ions with a larger mass and higher < Eion > , a high Hf(III) fraction of 0.86 is obtained and major suppression of in-grain nano-porosity is observed, in contrast to the H2 plasma process.
Experimental Section
Plasma-Assisted ALD of HfNx
The ALD of HfNx thin films was conducted in an Oxford Instruments FlexAL ALD reactor [14, 29, 30], equipped with an inductively coupled remote plasma (ICP) source with an alumina dielectric tube. The reactor chamber was pumped to a base pressure of 10–6 Torr using a turbo-molecular pump before every deposition. A stage temperature of 450 °C was selected for HfNx ALD. This corresponds to a substrate temperature of about 340 °C as verified by spectroscopic ellipsometry (SE) [13, 14]. The onset of precursor decomposition and the start of a CVD regime was observed above 450 °C as previously reported [14]. The reactor walls were kept at 145 °C during all the depositions.
The Hf precursor CpHf(NMe2)3 (Air Liquide, > 99.99% purity) was contained in a stainless steel bubbler at 60 °C and bubbled by an Ar flow of 100 sccm. An Ar flow of 100 sccm was also injected into the ICP alumina tube during the precursor dosage in order to suppress deposition on the ICP tube walls (a gate valve present between the ICP source and reactor chamber was kept open during the full cycle). Following the precursor dose, the chamber was pumped down to a base pressure of 10–6 Torr in order to remove the unreacted precursor and reaction byproducts for 4 s. For the plasma exposure half cycle, an Ar + H2 gas mixture (10 sccm Ar and 40 sccm H2) was introduced into the chamber from the ICP alumina tube. The valve to the pump was fully opened culminating in a reactor pressure of ~ 6 mTorr. After stabilization of the gas flows for 4 s, the plasma was ignited with 100 W radio frequency (rf) ICP power (13.56 MHz) for the desired time. The purge steps for both half cycles consisted of a flow of 200 sccm Ar through the bubbling lines in addition to the 100 sccm Ar flow from the ICP source and with the valve to the pump fully opened. A CpHf(NMe2)3 pulse length of 4 s and plasma exposure of 10 s were used, while keeping the purge step of 2 s after every half cycle [13].
External RF Substrate Biasing
Employing the special feature of our Oxford Instruments FlexAL systems, an external substrate bias is applied during the plasma half cycle for the last 5 s using an additional RF power source (13.56 MHz), attached to the substrate table [14]. As a result, a time-averaged negative substrate potential with respect to ground (Vbias) develops during the plasma exposure [14]. The magnitude of Vbias is tuned by changing the applied RF power as previously reported in detail (supporting information, Figure AI) [14]. An oscilloscope was connected to the substrate table via a high voltage probe that was used to measure the RF bias voltage waveforms as a function of time [14]. Supporting information Figure AI also shows the |Vbias| values for the applied RF bias power values [14, 31].
The ions are accelerated towards the surface of the growing film as a consequence of the voltage drop over the plasma sheath [31]. The ion flux-energy distribution functions (IFEDFs) of incident ions are measured using an Impedans Semion retarding field energy analyzer (RFEA) [31,32,33], as described in detail in Ref. [34]. As reported by Profijt et al., a mono-modal IFEDF for the grounded electrode (|Vbias|= 0 V) condition, whereas the application of external RF substrate bias resulted in a bi-modal IFEDF [31]. The shape of the IFEDF for a collisionless sheath is determined by the ratio of ion transit time to the period of RF cycle [32]. The mean ion energy is denoted by Eion, while ΔEion represents the peak-to-peak separation in a bimodal IFEDF in the text. Furthermore, the RFEA is also used to estimate the flux of incident ions using \({\Gamma }_{i}={{I}_{c}}\left/{e\cdot {A}_{e}}\right.\), where Ic is the collector current measured by the RFEA, e is the elementary charge and Ae is the effective collector area. Ref [34]. describes a detailed procedure to deduce the value of factor Ae, equal to (3.9 ± 1.0) 10–3 cm2 for the RFEA probe used in this work. Moreover, a slightly lower value of |Vbias| was obtained during the IFEDF measurements using RFEA than during the growth of HfNx films on a substrate, for a particular RF bias power application (supporting information, Figure AIc).
Substrates and Material Characterization
The HfNx films are deposited on planar Si(100) substrates with a diameter of 100 mm and with 450 nm SiO2 atop.
The growth per cycle (GPC) and the dielectric functions of the HfNx films were examined using spectroscopic ellipsometry (SE, J.A. Woollam, Inc., M2000U). The dielectric functions (0.75 ≤ hν ≤ 5.0 eV) of HfNx films could be modelled using one Drude and two Lorentz oscillators as demonstrated by Hu et al. (supporting information, Table AI for details and discussion on the selected optical model) [11]. Additionally, the optical film resistivity (ρop) was deduced from the free-carrier Drude parameterization, given by \({\rho }_{op}=({\Gamma }_{D}/{\varepsilon }_{o})\cdot {\omega }_{p}^{2}\), where ΓD is the damping factor for the Drude oscillator, εo is the permittivity of free space, and ωp is the screened plasma frequency. The electrical resistivity (ρe) was obtained via the four-point probe measurements using a Keithley 2400 SourceMeter and a Signaton probe by multiplying the sheet resistance of the HfNx films with the film thickness as derived from SE. All resistivities reported in this work are for room temperature.
X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermoscientific K-Alpha KA1066 system equipped with a monochromatic Al Kα (hν = 1486.6 eV) source in order to study the chemical bonding and the oxidation states of elements present in the film. The chemical composition and the mass density of the films was evaluated via Rutherford backscattering spectrometry (RBS) and elastic recoil detection (ERD) using 1900 keV4He+ ions (Detect99). For the ERD measurements, the recoil angle was 30° and the angle of incidence with the sample surface was kept at 15° whereas for the RBS, two detectors were used with scattering angles of 170° and 150°.
The surface morphology and the lateral grain sizes of thick HfNx films (t > 30 nm) were studied using a Zeiss Sigma field emission scanning electron microscope (FE-SEM) operated at an acceleration voltage of 2 kV.
The crystallinity of the HfNx films was examined with a PANalytical X’pert PRO MRD X-ray diffractometer using a Cu Kα (λ = 1.542 Ǻ) X-ray source. The X-ray diffractograms were obtained in a θ − 2θ configuration and were compared with the powder HfN patterns [35, 36]. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) studies were conducted using a JEOL ARM 200F operated at 200 kV in order to analyze: (1) the lateral grain sizes of thin HfNx films (t ≤ 10 nm), defined by the low atomic density grain boundary regions in the top-view images and (2) the microstructure and the nano-porosity of thick HfNx films (t > 30 nm), obtained from the cross-sectional samples. These cross-sectional samples were prepared using a Focused Ion Beam (FIB), following a standard lift-out sample preparation procedure. Prior to FIB milling, a protective layer was deposited on the HfNx layers. In case of the Ar–H2 plasma sample, a stack of Electron Beam Induced Deposited (EBID) SiO2, EBID Pt/C and Ion Beam Induced Deposited (IBID) Pt was used. In case of the H2 plasma sample, only EBID Pt/C and IBID Pt was used, as can be recognized from the TEM images below.
Results and Discussion
Ion Energy Characterization
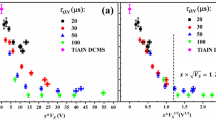
The IFEDF measurements were carried out at various values of |Vbias| for Ar–H2 plasma and are presented in Fig. 1. The IFEDFs for the H2 plasma process were also measured and are shown for comparison. It is noted that the energy distribution of ions with a specific mass cannot be resolved and rather a cumulative distribution of all the ions impinging on the surface is obtained. However, from literature [37,38,39] and a recent report from Sode et al. [28], it can be expected that an Ar–H2 plasma contains ArH+, Ar+, H3+, H2+ and H+ ions, with ArH+ being the most abundant ion under a similar experimental conditions. In a pure H2 plasma discharge, H3+ is the most abundant ion, in combination with H2+ and H+ ions [28].
A mono-modal IFEDF with an average ion energy (< Eion >) of 24 eV was obtained for the grounded electrode (|Vbias|= 0 V) for the Ar–H2 plasma as shown in Fig. 1a. The application of 10 W RF bias power resulted in an asymmetrical IFEDF with a |Vbias|= 102 V, < Eion > = 126 eV and an outermost peak-to-peak width (ΔEion) of 47 eV. Upon increasing the RF bias power to 60 W, the value of |Vbias| increased to 246 V, the < Eion > increased to 308 eV and an ΔEion of 77 eV was obtained. The increase in ΔEion with |Vbias| is a consequence of an increase in the peak-to-peak sheath voltage [32]. Furthermore, when closely inspecting the IFEDFs, they appear to be a superposition of multiple bi-modal IFEDFs of individual cationic species with different masses (i.e. ArH+, Ar+, H3+, H2+ and H+), that have different transit times through the plasma sheath as described by Manenschijn et al. [40]. This may be expected at low operating pressures used, where there are less ion-neutral collisions in the plasma sheath and ions are essentially unidirectional towards the surface of growing film.
Figure 1b shows the IFEDFs at various values of |Vbias| for the previously reported H2 plasma process at 30 mTorr. Similar to the Ar–H2 plasma, a mono-modal IFEDF was obtained for the grounded electrode (|Vbias|= 0 V), centered at < Eion > of 19 eV. Upon the application of external RF substrate bias, the IFEDF became bi-modal and an < Eion > of 98 eV with ΔEion of 39 eV was obtained at |Vbias|= 78 V (rf bias power = 10 W). Furthermore, the < Eion > and ΔEion increases with |Vbias| in a similar fashion as for Ar–H2 plasma.
The flux of impinging ions (Ґi) was calculated from the total ion current (Ic) recorded by the RFEA, since the flux of impinging ions is also expected to influence the film growth and related properties, as shown by Adibi et al. [41]. In our case, a constant Ґi of (9.0 ± 2.1)·1014 cm−2 s−1 was calculated independent of |Vbias| for the Ar–H2 plasma, whereas a slight increase in Ґi from (3.1 ± 0.7)·1014 cm−2 s−1 at |Vbias|= 0 V to (9.5 ± 2.2)·1014 cm−2 s−1 at |Vbias|= 173 V was observed for the H2 plasma process. Based on these results, we can conclude that the values of Ґi for both the processes are relatively similar in the entire range of |Vbias| investigated, whereas a significant increase in Eion is observed in the case of the Ar–H2 plasma.
Opto-Electrical Properties of HfNx
The ALD process was extensively characterized in terms of growth-per-cycle (GPC) and material properties. A detailed discussion can be found in the supporting information and here, only a few aspects are highlighted. The GPC was found to be constant at 0.35 ± 0.04 Å/cycle (supporting information Figure AIII), independent of the increase in the |Vbias| from 0 to 255 V. Above the |Vbias| value of 255 V, a slight increase in the GPC was observed. The previously reported data for the H2 plasma process is also included in supporting information Figure AIII for comparison. Furthermore, based on our previous studies on the ALD process employing H2 plasma, it is anticipated that the application of external RF substrate bias does not affect the saturation behavior of the ALD process [14].
Figure 2a shows the ρe and the ρop resistivity values for HfNx films as a function of |Vbias|. The HfNx films grown at |Vbias|= 0 V using an Ar–H2 plasma exhibit values of ρe of (2.0 ± 0.1) 10–1 Ωcm and of ρop = (5.2 ± 0.1) 10–3 Ωcm. When increasing the |Vbias| value up to 255 V, a substantial decrease in ρe to (4.1 ± 0.1) 10–4 Ωcm and in ρop to (2.4 ± 0.1) 10–4 Ωcm was observed. A subsequent increase in |Vbias| value to 367 V led to an increase in ρe to (6.5 ± 0.1)·10–4 Ωcm and ρop to (3.5 ± 0.1)·10–4 Ωcm. Figure 2a also contains the previously reported ρe and ρop data for the H2 plasma process for comparison [14].
a Electrical (ρe) and optical resistivity (ρop) values for ~ 35 nm HfNx films grown using Ar–H2 plasma compared with previously reported ~ 80 nm HfNx films grown using H2 plasma and b the corresponding Hf(III) oxidation state fractions as a function of |Vbias|. Lines serve as a guide to the eye and the (green) arrows indicate the optimum condition in terms of minimum in resistivity achieved in both ALD processes (Color figure online)
It should be noted that the very low ρe achieved at |Vbias|= 255 V is for HfNx films as thin as ~ 35 nm. To best of our knowledge, this resistivity value is the lowest reported for HfNx films grown by either CVD or ALD, and is comparable to values reported for PVD-grown films [13, 14, 21,22,23]. The |Vbias| value that yields the minimum in ρe and ρop is here referred to as optimum condition for the corresponding ALD process and the rest of the manuscript will address the film characterization mainly at the optimum conditions.
By considering that the interaction distance of the incident light with the HfNx films is rather small, it can be expected that only the crystalline quality within 3–4 nm is probed by SE for determining the ρop (see Table AI and the discussion underneath). Therefore, the difference between the ρe and ρop (Δρ) provides insights into the amount of electronic scattering in the HfNx films, as we previously described [13, 14, 42]. A very low Δρ is achieved at the optimum condition for Ar–H2 plasma. It is relevant here to underline that Δρ is an order of magnitude smaller than in the case of the previously reported H2 plasma process. This suggests that the application of Ar–H2 plasma has greatly contributed to reduce the amount of electronic scattering in the HfNx films. In order to comprehend the reason behind the very low scattering and the low value of ρe achieved at the optimum condition, the chemical composition and microstructure of the HfNx films was studied [14].
Chemical Properties of HfNx
The chemical composition of the HfNx films was investigated by means of XPS and RBS. The binding energy values attributed to each spectral line for a specific chemical element in the XPS spectrum can be found in the supporting information (Table AII). Figure 2b shows the Hf(III) oxidation state fraction as a function of |Vbias|. For comparison, the previously reported Hf(III) fraction data for H2 plasma is also shown. The Hf(III) fraction increased from 0.70 ± 0.02 to 0.86 ± 0.02 upon increasing the |Vbias| from 0 V up to the optimum condition (see the deconvolution of Hf 4f XPS spectrum in Figure AIVa-b). Interestingly, the high Hf(III) fraction achieved at the optimum condition for Ar–H2 plasma is comparable to the previously reported H2 plasma (i.e. 0.86 ± 0.02 vs. 0.82 ± 0.02).
Table 1 gives an overview of the material properties of HfNx films grown at the optimum condition using Ar–H2 plasma, whereas the film properties as a function of |Vbias| can be found in the supporting information (Table AIII). The aforementioned increase in Hf(III) fraction upon increasing the |Vbias| from 0 to 255 V is correlated with a major suppression in O content from 19.9 ± 0.9 at.% to < 2 at.%. In our previous report on H2 plasma [14], we also concluded that this increase in Hf(III) fraction with |Vbias| is associated with a major decrease in O content in the HfNx films. Additionally, the H content (and presumably Hf–H bonds) at the optimum condition appears to limit the Hf(III) fraction from reaching unity [14]. The latter suggestion is further corroborated by the fact that an increase in |Vbias| beyond the optimum condition leads to an increase in H at.%, while a simultaneous decrease in Hf(III) fraction is observed (Table AIII). It should be noted that the O content stays below < 2 at.% at |Vbias| of 255 V or higher. Furthermore, the C concentration was found to increase upon increasing the |Vbias| up to the optimum condition (see supporting information Figure AIVd). This increase in carbon is similar to previously reported H2 plasma process and is thought to arise from enhanced cracking of ligands on the film surface by energetic ions, followed by their re-deposition [14, 30].
Based on these results, it can be concluded that a high Hf(III) fraction and a very low O content is achieved at the optimum condition for the HfNx films grown using Ar–H2 plasma, which is comparable to the previously reported H2 plasma process.
Microstructural Properties of HfNx
The crystallinity and microstructure of the HfNx films were also investigated. In fact, we have previously shown that the ρe is also related to the film microstructure [14]. The crystallinity of 35 nm thick film grown using Ar–H2 plasma and 80 nm thick film grown using H2 plasma was examined using XRD (θ – 2θ mode) (supporting information, Figure AV). Conducting δ-HfN phase was observed for the optimum condition of the Ar–H2 plasma, exhibiting HfN(111), HfN(200) and HfN(220) reflections in a similar peak intensity ratio as the powder δ-HfN pattern [14], indicating no preferred growth direction.
The lateral grain sizes of the HfNx films were subsequently investigated. Figure 3 shows top-view high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and scanning electron microscopy (SEM) images of HfNx layers grown at the optimum condition of Ar–H2 plasma, allowing evaluation of the lateral grain size as a function of film thickness. The images for H2 plasma process are also shown for comparison purposes. It can be concluded from the data in HAADF-STEM and SEM images that the rate of lateral grain growth is higher for Ar–H2 plasma as compared to the H2 plasma (Fig. 4). Importantly, the lateral grain size for the relevant ~ 35 nm thick film grown using the Ar–H2 plasma is similar to that of previously reported ~ 80 nm thick HfNx film prepared with H2 plasma (green circles in Fig. 4).
Top-view high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and scanning electron microscopy (SEM) images for HfNx films prepared at the optimum conditions using Ar–H2 plasma and H2 plasma with an approximate thickness of (a, d) 10 nm; (b, e) 35 nm and 50 nm; (c,f) 90 nm and 80 nm, respectively
Lateral grain sizes for the HfNx films prepared at the optimum conditions of the Ar–H2 plasma and H2 plasma as a function of film thickness. The points with the (green) circles indicate the grain sizes of HfNx films that are discussed in more detail in terms of resistivity for the corresponding ALD process (Color figure online)
The in-grain crystal quality of the HfNx films grown at the optimum conditions was investigated by studying its micro-structure. Figure 5 shows the HAADF-STEM image of cross-sectional samples for the Ar–H2 plasma (35 nm thick) and the H2 plasma (80 nm thick) cases. Dark regions in the films indicate the presence of lighter elements and/or porosity. The images with a 50 nm scale display the lateral development of crystal grains as a function of height, yielding similar grain size values on the top surfaces of the film as measured from the aforementioned top-view HAADF-STEM and SEM studies of Fig. 3. The higher magnification insets reveal that the HfNx film grown using Ar–H2 plasma are quite dense, i.e. displaying much less nano-porosity than the case of H2 plasma. Specifically, non-uniform contrast variations and dark patches can be observed within the crystal grains of the HfNx film grown using H2 plasma, suggesting the presence of lower density regions such as nano-pores. These results agree with the higher mass density obtained for HfNx films grown using Ar–H2 plasma in comparison with the H2 plasma (Table 1).
Cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images for a ~ 35 nm thick film grown at |Vbias|= 255 V using Ar–H2 plasma and b ~ 80 nm thick film prepared using H2 plasma at |Vbias|= 130 V. The insets show magnified views of the micro-structure displaying the nano-scale porosity in the film as indicated by the highlighted (yellow) areas (Color figure online)
In addition, we observed the presence of V-shaped pyramidal voids for the Ar–H2 plasma in the initial phase of film growth (Fig. 5a). The cause for this phenomenon is not known yet. Although the voids can be observed in several areas of the whole TEM cross-section of the Ar–H2 sample (supporting information, Figure AVI), it appears that their presence does not affect the film electrical properties.
Discussion and Conclusions
Plasma-assisted ALD of HfNx has been studied using CpHf(NMe2)3 as the Hf(IV) precursor and Ar–H2 plasma in combination with an external RF substrate bias application as the co-reactant. Ion energy characterization reveals that the average energy of the impinging ions (< Eion >) on the HfNx surface at the optimum condition of the lowest film resistivity of 4.1 × 10–4 Ωcm for Ar–H2 plasma is 304 eV and the ion flux (Ґi) is (9.0 ± 2.1)·1014 cm−2 s−1. Such a low resistivity is achieved for films as thin as ~ 35 nm. From the extensive thin film characterization, we show that a very low O content (< 2 at.%) and a correlated high Hf(III) oxidation state fraction of 0.86 ± 0.02 is obtained for the HfNx films grown at the optimum condition. Furthermore, the HfNx films exhibit a very low in-grain nano-porosity. The high in-grain crystalline quality and low in-grain nano-porosity is also in line with the aforementioned observation on the very low electronic scattering (i.e. small Δρ) in the HfNx films (Fig. 2a). Altogether, these excellent material properties lead to the very low ρe of the HfNx films grown using Ar–H2 plasma with energetic ion bombardment.
Impinging ions during plasma processes are known to initiate several interactions with the growing films, for example, enhanced ad atom surface diffusion leading to decrease in the defect density; bulk lattice atom displacements resulting in a collision cascade; sputtering and ion-induced damage; ion implantation at higher ion energies [20, 43,44,45,46,47,48,49,50,51]. In regards to the material properties, Hultman et al. and Petrov et al. showed that energetic ions in a N2 and Ar–N2 plasma discharges respectively with |Vbias| value in the range of 150 V—250 V can lead to annihilation of defects and reduction in nano-porosity in sputtered TiN films [52, 53], and attributed the decrease in defect density to the near-surface and sub-surface diffusion processes [44, 45].
Next to the ion energy, the mass of impinging ions is of importance. In our case, from the ion energy results and from literature reports [28], it is concluded that the HfNx film surface at the optimum conditions is subjected to energetic ions with significantly higher ion energy and larger mass when using an Ar–H2 plasma (mainly ArH+) instead of a H2 plasma (< Eion > = 159 eV, mainly H3+), while the Ґi remains comparable (Ґi = (8.2 ± 1.9)·1014 cm−2 s−1 for H2 plasma). It is further noted that the ρe value for HfNx films grown at the optimum conditions using Ar–H2 plasma is an order of magnitude lower than the H2 plasma. This major decrease in the ρe value is primarily attributed to the improvement in the HfNx film microstructure, enabled by the impingement of ions with a larger mass and higher energy. Relevantly, Villamayor et al. also recently showed that an increase in the mass of impinging ions by adopting Kr–N2 plasma, instead of Ar–N2 plasma, led to an improvement in the crystalline quality of the sputtered HfNx films [20]. The above results can be understood from the fact that the nature of ion-surface interaction depends on the extent of energy and momentum transfer. As highlighted by Gago et al. [48], the system can be approximated by the simple case of an elastic binary atom(ion)-atom collision. Using this model, the kinematic factor (k) for energy transfer can be easily calculated using \(k={4Mm}\left/ {{\left(M+m\right)}^{2}}\right.\cdot {cos}^{2}\varphi\), where M and m are the masses of incoming ion and target atom respectively and φ is the scattering angle [48]. It should be noted that the value of k is approximately an order of magnitude higher for the impinging heavy ArH+ ions in comparison to the light H3+ ions, when Hf is considered as the target atom. Altogether, it is expected that the impinging ions with a higher energy (304 vs. 159 eV) and larger mass (ArH+ vs. H3+) may lead to a greater extent of energy and momentum transfer to the HfNx film surface [20, 43,44,45,46,47,48,49,50,51].
In addition to the higher energy and larger momentum transfer of ArH+ ions, it should also be noted that ArH+ ions are expected to have a smaller penetration depth than H3+ ions. This signifies that the energy and momentum transfer per ALD cycle using ArH+ ions occurs primarily in a shallower region of the film. Therefore, the energy density available to promote the decrease in nanoporosity is higher when using ArH+ ions. So to conclude, the obtained results demonstrate how energy density and mass of impinging ions and the associated energy and momentum transfer during plasma ALD can contribute to the fine tuning of the chemical and microstructural properties of HfNx thin films. The results may be applicable to wide range of ALD processes including for the growth of other transition metal nitrides [54].
Supporting Information
Substrate potential via oscilloscope, GPC as a function of |Vbias|, SE modeling parameters for HfNx, HfNx material property as a function of |Vbias|, XPS spectra for Hf 4f, O 1 s and C 1 s as a function of |Vbias|, θ-2θ XRD scan and HAADF-STEM image for HfNx film strip.
References
Liu Y, Nabatame T, Nguyen N, Matsukawa T, Endo K, O'Uchi S, Tsukada J, Yamauchi H, Ishikawa Y, Mizubayashi W, Morita Y, Migita S, Ota H, Chikyow T, Masahara M (2015) Channel shape and interpoly dielectric material effects on electrical characteristics of floating-gate-type three-dimensional fin channel flash memories. Jpn J Appl Phys. https://doi.org/10.7567/JJAP.54.04DD04
Pierson HO (1996) Handbook of Refractory Carbides and Nitrides: Properties, Characteristics Processing and Apps. Elsevier Science, Amsterdam
Franklin AD, Koswatta SO, Farmer DB, Smith JT, Gignac L, Breslin CM, Han S-J, Tulevski GS, Miyazoe H, Haensch W, Tersoff J (2013) Carbon s. Nano Lett 13(6):2490–2495. https://doi.org/10.1021/nl400544q
Yu HY, Li MF, Kwong DL (2004) Thermally robust HfN metal as a promising gate electrode for advanced MOS device applications. IEEE Trans Electron Devices 51(4):609–615. https://doi.org/10.1109/TED.2004.825110
Hu CK, Harper JME (1998) Copper interconnections and reliability. Mater Chem Phys 52(1):5–16. https://doi.org/10.1016/S0254-0584(98)80000-X
Jeong W, Ko Y, Bang S, Lee S, Jeon H (2010) Characteristics of HfN films deposited by using remote plasma-enhanced atomic layer deposition. J Korean Phys Soc 56(3):905–910
Kim H, Cabral C, Lavoie C, Rossnagel SM (2002) Diffusion barrier properties of transition metal thin films grown by plasma-enhanced atomic-layer deposition. J Vac Sci Technol B Microelectron Nanometer Struct 20(4):1321–1326. https://doi.org/10.1116/1.1486233
Yu HY, Kang JF, Ren C, Chen JD, Hou YT, Shen C, Li MF, Chan DSH, Bera KL, Tung CH, Kwong DL (2004) Robust high-quality HfN-HfO 2 Gate stack for advanced MOS device applications. IEEE Electron Device Lett 25(2):70–72. https://doi.org/10.1109/LED.2003.820649
Xu M, Wang S, Yin G, Li J, Zheng Y, Chen L, Jia Y (2006) Optical properties of cubic Ti3N4, Zr3N4, and Hf3N4. Appl Phys Lett. https://doi.org/10.1063/1.2360937
Becker JS, Kim E, Gordon RG (2004) Atomic layer deposition of insulating hafnium and zirconium nitrides. Chem Mater 16(18):3497–3501. https://doi.org/10.1021/cm049516y
Hu C, Gu Z, Wang J, Zhang K, Zhang X, Li M, Zhang S, Fan X, Zheng W (2014) Nature of tunable optical reflectivity of rocksalt hafnium nitride films. J Phys Chem C 118(35):20511–20520. https://doi.org/10.1021/jp504004e
Farrell IL, Reeves RJ, Preston ARH, Ludbrook BM, Downes JE, Ruck BJ, Durbin SM (2010) Tunable electrical and optical properties of hafnium nitride thin films. Appl Phys Lett. https://doi.org/10.1063/1.3327329
Karwal S, Williams BL, Niemelä JP, Verheijen MA, Kessels WMM, Creatore M (2017) Plasma-assisted atomic layer deposition of HfNx: Tailoring the film properties by the plasma gas composition. J Vac Sci Technol A Vac Surf Films. https://doi.org/10.1116/1.4972208
Karwal S, Verheijen MA, Williams BL, Faraz T, Kessels WMM, Creatore M (2018) Low resistivity HfNx grown by plasma-assisted ALD with external RF substrate biasing. J Mater Chem C 6(15):3917–3926. https://doi.org/10.1039/C7TC05961B
Knoops HCM, Langereis E, Sanden MCMVD, Kessels WMM (2012) Reaction mechanisms of atomic layer deposition of TaNx from Ta(NMe2)5 precursor and H2-based plasmas. J Vac Sci Technol, A 30(1):01A101. https://doi.org/10.1116/1.3625565
Johansson BO, Helmersson U, Hibbs MK, Sundgren JE (1985) Reactively magnetron sputtered Hf-N films. I. Composition and structure. J Appl Phys 58(8):3104–3111. https://doi.org/10.1063/1.335812
Johansson BO, Sundgren JE, Helmersson U (1985) Reactively magnetron sputtered Hf-N films. II. Hardness and electrical resistivity. J Appl Phys 58(8):3112–3117. https://doi.org/10.1063/1.335813
Seo HS, Lee TY, Wen JG, Petrov I, Greene JE, Gall D (2004) Growth and physical properties of epitaxial HfN layers on MgO(001). J Appl Phys 96(1):878–884. https://doi.org/10.1063/1.1759783
Shinkai S, Sasaki K (1999) Influence of sputtering parameters on the formation process of high-quality and low-resistivity HfN thin film. Jpn J Appl Phys Part 1 Regul Pap Short Note Rev Pap 38(4):2097–2102
Villamayor MMS, Keraudy J, Shimizu T, Viloan RPB, Boyd R, Lundin D, Greene JE, Petrov I, Helmersson U (2018) Low temperature (Ts/Tm %3c 0.1) epitaxial growth of HfN/MgO(001) via reactive HiPIMS with metal-ion synchronized substrate bias. J Vac Sci Technol A 36(6):061511. doi:10.1116/1.5052702
Kim Y, Baunemann A, Parala H, Devi A, Fischer RA (2005) Metal-organic CVD of conductive and crystalline hafnium nitride films. Chem Vapor Deposition 11(6–7):294–297. https://doi.org/10.1002/cvde.200504204
Consiglio S, Zeng W, Berliner N, Eisenbraun ET (2008) Plasma-assisted atomic layer deposition of conductive hafnium nitride using tetrakis(ethylmethylamino)hafnium for CMOS gate electrode applications. J Electrochem Soc 155(3):H196–H201. https://doi.org/10.1149/1.2827995
Kim EJ, Kim DH (2006) Highly conductive HfN x films prepared by plasma-assisted atomic layer deposition. Electrochem Solid State Letters 9(8):C123–C125. https://doi.org/10.1149/1.2206884
Elam JW, Libera JA, Huynh TH, Feng H, Pellin MJ (2010) Atomic layer deposition of aluminum oxide in mesoporous silica gel. J Phys Chem C 114(41):17286–17292. https://doi.org/10.1021/jp1030587
Haukka S, Lakomaa E-L, Julha O, Vilhunen J, Hornytzkyj S (1993) Dispersion and distribution of titanium species bound to silica from TiCl4. Langmuir 9(12):3497–3506
Lakomaa EL, Root A, Suntola T (1996) Surface reactions in Al2O3 growth from trimethylaluminium and water by atomic layer epitaxy. Appl Surf Sci 107:107–115. https://doi.org/10.1016/s0169-4332(96)00513-2
Keranen J, Guimon C, Iiskola E, Auroux A, Niinisto L (2003) Surface-controlled gas-phase deposition and characterization of highly dispersed vanadia on silica. J Phys Chem B 107(39):10773–10784
Sode M, Schwarz-Selinger T, Jacob W (2013) Ion chemistry in H2-Ar low temperature plasmas. J Appl Phys 114 (6). https://doi.org/10.1063/1.4817526
Knoops HCM, Braeken EMJ, De Peuter K, Potts SE, Haukka S, Pore V, Kessels WMM (2015) Atomic layer deposition of silicon nitride from Bis(tert-butylamino)silane and N%3cinf%3e2%3c/inf%3e Plasma. ACS Appl Mater Interfaces 7(35):19857–19862. https://doi.org/10.1021/acsami.5b06833
Knoops HCM, De Peuter K, Kessels WMM (2015) Redeposition in plasma-assisted atomic layer deposition: silicon nitride film quality ruled by the gas residence time. Appl Phys Lett. https://doi.org/10.1063/1.4926366
Profijt HB, Van De Sanden MCM, Kessels WMM (2013) Substrate-biasing during plasma-assisted atomic layer deposition to tailor metal-oxide thin film growth. J Vac Sci Technol A Vac Surf Films. https://doi.org/10.1116/1.4756906
Gahan D, Daniels S, Hayden C, Scullin P, O'Sullivan D, Pei YT, Hopkins MB (2012) Ion energy distribution measurements in RF and pulsed dc plasma discharges. Plasma Sour Sci Technol. https://doi.org/10.1088/0963-0252/21/2/024004
Gahan D, Dolinaj B, Hopkins MB (2008) Retarding field analyzer for ion energy distribution measurements at a radio-frequency biased electrode. Rev Sci Instrum. https://doi.org/10.1063/1.2890100
Faraz T, Arts K, Karwal S, Knoops HCM, Kessels WMM (2019) Energetic ions during plasma-enhanced atomic layer deposition and their role in tailoring material properties. Plasma Sour Sci Technol 28(2):024002. https://doi.org/10.1088/1361-6595/aaf2c7
Timofeeva II, Shvedova LK (1972) Microhardness and thermal expansion of transition metal nitrides within the 80–300K temperature range. Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy 8(6):1169–1170
Wang W, Nabatame T, Shimogaki Y (2008) Preparation of conductive HfN by post rapid thermal annealing-assisted MOCVD and its application to metal gate electrode. Microelectron Eng 85(2):320–326. https://doi.org/10.1016/j.mee.2007.07.003
Rennick CJ, Engeln R, Smith JA, Orr-Ewing AJ, Ashfold MNR, Mankelevich YA (2005) Measurement and modeling of a diamond deposition reactor: hydrogen atom and electron number densities in an Ar∕H2 arc jet discharge. J Appl Phys 97(11):113306. https://doi.org/10.1063/1.1906288
Meulenbroeks RFG, van Beek AJ, van Helvoort AJG, van de Sanden MCM, Schram DC (1994) Argon-hydrogen plasma jet investigated by active and passive spectroscopic means. Phys Rev E 49(5):4397–4406. https://doi.org/10.1103/PhysRevE.49.4397
Jiménez-Redondo M, Cueto M, Doménech JL, Tanarro I, Herrero VJ (2014) Ion kinetics in Ar/H2 cold plasmas: the relevance of ArH+. RSC advances 4(107):62030–62041. https://doi.org/10.1039/C4RA13102A
Manenschijn A, Janssen GCAM, Van Der Drift E, Radelaar S (1991) Measurement of ion impact energy and ion flux at the RF electrode of a parallel plate reactive ion etcher. J Appl Phys 69(3):1253–1262. https://doi.org/10.1063/1.347311
Adibi F, Petrov I, Greene JE, Hultman L, Sundgren JE (1993) Effects of high-flux low-energy 20–100 eV ion irradiation during deposition on the microstructure and preferred orientation of Ti0.5Al0.5N alloys grown by ultra-high-vacuum reactive magnetron sputtering. J Appl Phys 73(12):8580–8589. https://doi.org/10.1063/1.353388
Knoops HCM, van de Loo BWH, Smit S, Ponomarev MV, Weber J-W, Sharma K, Kessels WMM, Creatore M (2015) Optical modeling of plasma-deposited ZnO films: Electron scattering at different length scales. J Vac Sci Technol A 33(2):021509. https://doi.org/10.1116/1.4905086
Insepov Z, Yamada I, Sosnowski M (1998) Sputterring and smoothing of metal surface with energetic gas cluster beams. Mater Chem Phys 54(1):234–237. https://doi.org/10.1016/S0254-0584(98)00032-7
Wang LP, Fu KY, Tian XB, Tang BY, Chu PK (2002) Influence of temperature and ion kinetic energy on surface morphology of CeO2 films prepared by dual plasma deposition. Mater Sci Eng, A 336(1):75–80. https://doi.org/10.1016/S0921-5093(01)01923-2
Gottlieb SO, Satoshi H (2018) Foundations of low-temperature plasma enhanced materials synthesis and etching. Plasma Sour Sci Technol 27(2):023001
Nastasi M, Mayer J, Hirvonen JK (1996) Ion stopping. In: Ion-solid interactions: fundamentals and applications. Cambridge Solid State Science Series. Cambridge University Press, Cambridge, pp 88–114. https://doi.org/10.1017/CBO9780511565007.006
Insepov Z, Yamada I (1995) Molecular dynamics simulation of cluster ion bombardment of solid surfaces. Nucl Instrum Methods Phys Res, Sect B 99(1):248–252. https://doi.org/10.1016/0168-583X(95)00322-3
Gago R, Jiménez I, Albella J (2006) Thin film growth by ion-beam-assisted deposition techniques. Elesiver, Amsterdam, pp 345–382. https://doi.org/10.1016/B978-008044496-3/50011-3
Anders A (2010) A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Films 518(15):4087–4090. https://doi.org/10.1016/j.tsf.2009.10.145
Auciello O, Kelly R (1984) Ion bombardment modification of surfaces: fundamentals and applications. Elsevier Science Publishers, Amsterdam
Greene JE, Barnett SA (1982) Ion-surface interactions during vapor phase crystal growth by sputtering, MBE, and plasma-enhanced CVD: applications to semiconductors. J Vac Sci Technol V 21(2):285–302
Hultman L, Helmersson U, Barnett SA, Sundgren JE, Greene JE (1987) Low-energy ion irradiation during film growth for reducing defect densities in epitaxial TiN(100) films deposited by reactive-magnetron sputtering. J Appl Phys 61(2):552–555. https://doi.org/10.1063/1.338257
Petrov I, Hultman L, Helmersson U, Sundgren JE, Greene JE (1989) Microstructure modification of TiN by ion bombardment during reactive sputter deposition. Thin Solid Films 169(2):299–314. https://doi.org/10.1016/0040-6090(89)90713-X
Faraz T, Knoops HCM, Verheijen MA, van Helvoirt CAA, Karwal S, Sharma A, Beladiya V, Szeghalmi A, Hausmann DM, Henri J, Creatore M, Kessels WMM (2018) Tuning material properties of oxides and nitrides by substrate biasing during plasma-enhanced atomic layer deposition on planar and 3D substrate topographies. ACS Appl Mater Interfaces 10(15):13158–13180. https://doi.org/10.1021/acsami.8b00183
Acknowledgments
The authors thank Dr. Vincent Vandalon, Dr. Richard Engeln and Dr. Harm Knoops (University of Technology Eindhoven) for fruitful discussions and Cristian van Helvoirt and Jeroen van Gerwen (University of Technology Eindhoven) for the skillful technical assistance. Dr. Beatriz Barcones Campo (University of Technology Eindhoven) is acknowledged for the FIB preparation of the TEM samples. Solliance and the Dutch province of Noord-Brabant are acknowledged for funding the TEM facility. The authors would also like to thank Vivek Beladiya (University of Jena) for the stress measurements. This work was supported financially by Technologiestichting STW through the project LIMIET (#13316) and Netherlands Organization for Scientific Research NWO through project HTSM (#15352). Air Liquide is kindly acknowledged for providing the Hf precursor.
Author information
Authors and Affiliations
Contributions
All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karwal, S., Verheijen, M.A., Arts, K. et al. Plasma-Assisted ALD of Highly Conductive HfNx: On the Effect of Energetic Ions on Film Microstructure. Plasma Chem Plasma Process 40, 697–712 (2020). https://doi.org/10.1007/s11090-020-10079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10079-x