Abstract
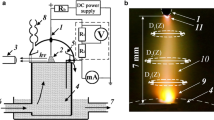
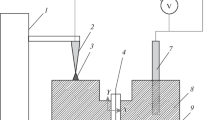
The transfer of solvent and components of dissolved substances from aqueous solutions to the gas phase under the action of atmospheric pressure air glow discharge was experimentally studied. solutions of NaCl, KCl, CuCl2, MgCl2, CaCl2, SrCl2, BaCl2, NaNO3, KNO3, Ba(NO3)2, Na2SO4, K2SO4, CuSO4 with concentrations of 0.1–0.5 mol/L were used as the cathodes of DC discharge at a current of 10–70 mA. The influence of the solution composition on cathode voltage drop and the electric field strength in plasma has been shown. Plasma emission spectra showed the appearance of metal atoms in the plasma requires threshold discharge current or threshold power input to the liquid cathode by ion bombardment. The threshold power values depend on the mass of hydrated cations and their concentration in solution. The efficiency of the transfer processes was characterized by transfer coefficients—the number of particles transferred from the liquid to the gas phase per one ion bombarding the cathode. Dependences of the transfer coefficients on the power dissipated in the cathode region and on the hydration energy of the cations were obtained. Experimental data on the rate of condensate accumulation in the special trap were used to estimate the concentrations of water molecules in the plasma.











Similar content being viewed by others
References
Bo Jiang, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, Xue Q (2012) Chem Eng J 236:348
Du CM, Wang J, Zhang L, Li HX, Liu H, Xiong Y (2012) New J Phys 14:013010
Yang Y, Cho YI, Fridman A (2012) Plasma discharge in liquid: water treatment and applications. CRC Press, New York
Titov VA, Shikova TG, Rybkin VV, Ageeva TA, Choi HS (2006) High Temp Mater Process 10:467
Choi HS, Shikova TG, Titov VA, Rybkin VV (2006) J Coll Interface Sci 300:640
Titov VA, Rybkin VV, Shikova TG, Ageeva TA, Golubchikov OA, Choi HS (2005) Surf Coat Technol 199:231
Mariotti D, Sankaran RM (2010) J Phys D Appl Phys 43:323001
Saito G, Akiyama T (2015) J Nanomater 2015:123696
Takai O (2008) Pure Appl Chem 80:2003
Webb MR, Andrade FJ, Gamez G, McCrindle R, Hieftje GM (2005) J Anal At Spectrom 20:1218
Mezei P, Cserfalvi T (2007) Appl Spectrosc Rev 42:573
Bencs L, Laczai N, Mezei P, Cserfalvi T (2015) Spectrochim Acta, Part B 107:139
Yang C, Wang L, Zhu Z, Jin L, Zheng H, Belshaw NS, Hu S (2016) Talanta 155:314
Bruggeman PJ, Kushner MJ, Locke BR et al (2016) Plasma Sources Sci Technol 25:053002
Bruggeman P, Leys C (2009) J Phys D Appl Phys 5:053001
Titov VA, Rybkin VV, Maximov AI, Choi HS (2005) Plasma Chem Plasma Process 25:503
Mezei P, Cserfalvi T (2012) Sensors 12:6576
Maximov AI, Khlustova AV (2007) High Temp Mater Process 11:527
Verreycken T, Schram DC, Leys C, Bruggeman P (2010) Plasma Sources Sci Technol 19:045004
Kutepov AM, Zakharov AG, Maksimov AI, Titov VA (2003) High Energy Chem 37:362
Khlyustova AV, Sirotkin NA, Maximov AI (2010) High Energy Chem 44:75
Sirotkin NA, Titov VA (2016) Plasma Phys Tech 3:126
Cserfalvi T, Mezei P (2005) J Anal At Spectrom 20:939
Maksimov AI, Titov VA, Khlyustova AV (2004) High Energy Chem 38:196
Webb MR, Andrade FJ, Hieftje GM (2007) Anal Chem 79:7807
Shirai N, Matsui K, Ibuka S, Ishii S (2007) IEEE Trans Plasma Sci 39:2210
Sirotkin NA, Titov VA (2017) J Phys: Conf Ser 789:012054
Ecker G, Emeleus KG (1954) Proc Phys Soc Sec B 67:546
Khlyustova AV, Dydykin MG, Maksimov AI, Polyakov MS (2008) Surf Eng Appl Electrochem 44:370
Holler FJ, Skoog DA, West DM (1996) Fundamentals of analytical chemistry. Saunders College Pub, Philadelphia
Titov VA, Rybkin VV, Smirnov SA, Kulentsan AI, Choi HS (2006) Plasma Chem Plasma Process 26:543
Maxwell KL, Hudson MK (2005) J. Pyrotech. 21:59
Maehler J, Persson I (2011) Inorg Chem 51:425
Maximov AI, Kuzmicheva LA, Khlustova AV, Titova YV, Dydykin MG (2007) Mendeleev Commun 17:294
Schwartz AJ, Ray SJ, Elish E, Storey AP, Rubinshtein AA, Chan GCY, Hieftje GM (2012) Talanta 102:26
Nikiforov AY (2008) High Energy Chem 43:235
Sirotkin NA, Titov VA (2016) Appl Phys 6:25
Maksimov AI, Khlyustova AV (2009) High Energy Chem 43:51
Shirai N, Ichinose K, Uchida S, Tochikubo F (2011) Plasma Sources Sci Technol 20:034013
Bobkova E, Smirnov S, Zalipaeva Y, Rybkin V (2014) Plasma Chem Plasma Process 34:721
Rybkin VV, Smirnov SA, Titov VA, Arzhakov DA (2010) High Temp 48:476
Sirotkin NA, Khlyustova AV, Maksimov AI (2014) Surf Eng Appl Electrochem 50:323
Manion JA, Huie RE, Levin RD et al (2015) NIST chemical kinetics database, NIST standard reference database 17, Version 7.0 (Web Version), Release 1.6.8, Data version 2015.12. Gaithersburg, Maryland, pp 20899–8320. http://kinetics.nist.gov/
Plasma Data Exchange Project: http://fr.lxcat.net. Retrieved 7 July 2017
Acknowledgements
The study was supported by Russian Foundation for Basic Research according to the research project 16–33–60061 mol_a_dk.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirotkin, N.A., Titov, V.A. Transfer of Liquid Cathode Components to the Gas Phase and Their Effect on the Parameters of the Atmospheric Pressure DC Discharge. Plasma Chem Plasma Process 37, 1475–1490 (2017). https://doi.org/10.1007/s11090-017-9840-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9840-8