Abstract
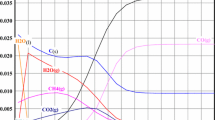
This work deals with incineration of organic liquid wastes using an oxygen thermal plasma jet, submerged in water. The results presented here concern incineration of trichloroethylene (TCE). During a trial run, the CO2 and CO content in the exhaust gas is continuously measured; samples taken periodically from the solution are analyzed by appropriate methods: total organic carbon and chlorine content are measured. Process efficiency during tests with a few L/h of TCE is given by the mineralization rate. The trapping rate of chlorine as HCl is near 100 %. The TCE destruction and removal efficiency, measured by MS/GC, is better than 99.9999 %. A simplified kinetic model of gas quenching was constructed from a single-phase plug-flow reactor model taking into account 14 species and 34 reactions. It satisfies the requirements of heat balance and major components analysis, and reveals the major role of the OH radical on the concentrations of CO as well as HCl and/or Cl2 in the off-gas stream.


















Similar content being viewed by others
References
Lemont F, Lafon C, Baronnet JM, Boudesocque N (2013) Method and device for the treatment of waste through injection into an immersed plasma, EP2507556. http://www.google.com.na/patents/EP2507556B1?hl=fr&cl=en
Mabrouk M, Marchand M, Russello A, Baronnet JM, Lemont F (2015) Development of a submerged thermal plasma process for combustion of organic liquid waste. Plasma Chem Plasma Process 35(1):45–60
INRS (2011) Trichloroéthylène Fiche toxilogique no 22. Institut National de Recherche et de Sécurité. http://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_22
Bose D, Senkan SM (1983) On the combustion of chlorinated hydrocarbons. I: Trichloroethylene. Anglais 35(1–4):187–202
Chang WD, Karra SB, Senkan SM (1986) Molecular beam mass spectroscopic study of trichloroethylene flames. Environ Sci Technol 20(12):1243–1248
Chang WD, Senkan SM (1989) Detailed chemical kinetic modeling of fuelrich C2HCl3/O2/Ar flames. Environ Sci Technol 23(4):442–450
Senkan SM (2000) Survey of rate coefficients in the C–H–Cl–O system. In: Gardiner WC Jr (ed). Gas Phase Combust Chem 389–487. Springer, New York
Baronnet JM, Lemont F, Mabrouk M, Marchand M (2014) Aménagement de la tuyère de sortie d’une torche à plasma immergé dédiée au traitement de déchets, WO 2015165911 A1
Chloride cuvette test. http://www.hach-lange.co.uk/chloride-cuvette-test-1-70-mg-l-70-1000-mg-l-cl/product?id=26370291406
Baronnet JM, Sanon A, Debbagh Nour G (1989) ALEX: Un code de calcul des propriétés thermodynamiques et des coefficients de transport des jets de plasma thermique, DOPEE 85. Procédés Electriques dans des traitements de surface, Paris
Chase MW (1998) NIST-JANAF thermochemical tables, journal of physical and chemical reference data, 4th edn. American Chemical Society, Washington, DC; American Institute of Physics for the National Institute of Standards and Technology, Woodbury, NY
GRI_MECH (1998) GRI-Mech 3.0. http://www.me.berkeley.edu/gri_mech/
Fisher EM, Koshland CP (1990) Numerical simulation of the thermal destruction of some chlorinated C1 and C2 hydrocarbons, vol 40. Air & Waste Management Association, Pittsburgh
Werner JH, Cool TA (2000) The kinetics of the combustion of trichloroethylene for low Cl/H ratios. Combust Flame 120(1–2):125–142
Baulch DL, Duxbury J, Grant SJ, Montague DC (1981) Homogeneous gas phase reactions of halogen- and cyanide-containing species. J Phys Chem Ref Data 10:723
Kee RJ, Lutz AE, Miller JA (1988) SENKIN: a FORTRAN program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis, Sandia National Laboratories Report 87–8248
Brilhac JF (1993) Contribution à l’étude statique et dynamique de torches plasma stabilisées par vortex. Thesis, Université de Limoges
Alekseev NV, Pozdnyakov OE, Shorin SN (1983) Study of the interaction between a hot gas jet and a liquid bath. J Eng Phys Thermophys 44(4):358–363
Warnatz J (1984) Combustion chemistry. In: Gardiner WC Jr (ed) pub. Springer, New York
Tsang W, Hampson RF (1986) Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J Phys Chem Ref Data 15:1087–1279
Natarajan K, Roth P (1987) High temperature rate coefficient for the reaction of O(3P) with H2 obtained by the resonance absorption of O and H atoms. Combust Flame 70:267–279
Frenklach M, Wang H, Rabinowitz MJ (1992) Optimization and analysis of large chemical kinetic mechanisms using the solution mapping method—combustion of methane. Prog Energy Combust Sci 18:47
Davidson DF, Petersen EL, Rohrig M, Hanson RK (1987) Measurement of the rate coefficient of H + O2 → HO2 + M for M = Ar and N2 at high pressures. Paper presented at the 26th Symposium (Int’l.) on Combustion. p 481
Yu CL, Frenklach M, Masten DA, Hanson RK, Bowman CT (1994) Reexamination of shock-tube measurements of the rate coefficient of H + O2 → OH + O. J Phys Chem Ref Data 98:4770–4771
Michael JV, Sutherland JW (1988) Rate constant for the reaction of H with H2O and OH with H2 by the flash photolysis-shock tube technique over the temperature range 1246–2297 K. J Phys Chem 92(13):3853–3857
Baulch DL, Cobos CJ, Cox RA, Esser C, Frank P, Walker RW, Warnatz J (1992) Evaluated kinetic data for combustion modelling. J Phys Chem Ref Data 21:411
Wooldridge MS, Hanson RK, Bowman CT (1994) A shock tube study of the OH + OH → H2O + O reaction. Int J Chem Kinet 26:389
Keyser LF (1988) Kinetics of the reaction OH + HO2 → H2O + O2. J Phys Chem 92:1193
Yu CL, Wang C, Franklach M (1990) Measurement of the rate coefficient of reaction CO + OH → CO2 + H. Paper presented at the Meeting of the Eastern States Section/Combustion Institute, Orlando
Hippler H, Troe J, Willner J (1990) Shock wave study of the reaction HO2 + HO → H2O2 + O2. J Chem Phys 93:1755
Baulch DL, Drysdale DD, Duxbury J, Grant SJ (1976) Evaluated kinetic data for high temperature reactions, vol. 3. Butterworths, London, p 595
Mabrouk M, Lemont F, Baronnet JM (2012) Incineration of radioactive organic liquid wastes by underwater thermal plasma. J Phys: Conf Ser 406(1):12002–12009
Acknowledgments
The pharmacology, toxicology and pharmacovigilance department of the Limoges University Hospital is gratefully acknowledged for performing analysis of the solution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mabrouk, M., Marchand, M., Baronnet, JM. et al. Trichloroethylene Combustion in a Submerged Thermal Plasma: Results and Chemical Kinetics Model. Plasma Chem Plasma Process 36, 1085–1110 (2016). https://doi.org/10.1007/s11090-016-9693-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9693-6