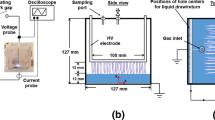
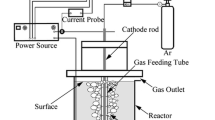
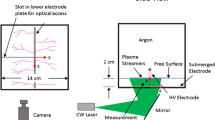
Abstract
The effect of pulsed high-voltage discharge on mass transfer and reaction processes in a needle-plate reactor was studied. Bubble size distributions and characteristic parameters were determined by photography, implying that inlet air bubbles could be broken into smaller ones effectively by discharge. The mass transfer parameters including specific interfacial area (a), volumetric mass transfer coefficient (k L a), and liquid-side mass transfer coefficient (k L) were obtained by Danckwerts-plot, reflecting the mass transfer processes. In addition, 4-chlorophenol (4-CP) decomposition was determined under the same experimental conditions. The results showed that the conclusions of 4-CP removal were coincided with the statistical results of bubble images, illustrating that higher peak voltage, pulsed frequency and appropriate air flow rate are beneficial to mass transfer and reaction. However, there was a slight difference between 4-CP removal and mass transfer parameters at higher air flow rate. Therefore, it is more reasonable to optimize the operating conditions of a pulsed high-voltage discharge reactor via combining image acquisition and mass transfer parameters with the results of pollutant degradation.













Similar content being viewed by others
References
Sun B, Sato M, Clements JS (1999) Use of a pulsed high-voltage discharge for removal of organic compounds in aqueous solution. J Phys D Appl Phys 32:1908–1915
Joshi AA, Locke BR, Arce P, Finney WC (1995) Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater 41:3–30
Sato M, Ohgiyama T, Clements JS (1996) Formation of chemical specie and their effects on microorganisms using a pulsed high-voltage discharge in water. IEEE Trans Ind Appl 32:106–112
Sun B, Sato M, Clements JS (1997) Optical study of active species produced by a pulsed streamer corona discharge in water. J Electrostat 39:189–202
Sun B, Sato M, Clements JS (2000) Oxidative processes occurring when pulsed high voltage discharges degrade phenol in aqueous solution. Environ Sci Technol 34:509–513
Lu X, Liu K, Liu M, Pa Y, Zhang H (2001) Research on high density plasma of pulsed discharge in water. Pulsed Power Plasma Sci 2:1595–1598
Hemmert D, Shiraki K, Yokoyama T, Katsuki S, Bluhm H, Akiyama H (2003) Optical diagnostics of shock waves generated by a pulsed streamer discharge in water. 14th IEEE Int Pulsed Power Conf 1:232–235
Clements JS, Sato M, Davis RH (1987) Preliminary investigation of prebreakdown phenomena and chemical reactions using a pulsed high voltage discharge in water. IEEE Trans Ind Appl IA 23:224–235
Sunka P, Babicky V, Clupek M, Fuciman M, Schmidt J, Benes J (2001) Generation of focused shock waves by multi-channel discharges in water. Pulsed Power Plasma Sci 2:1134–1137
Piskarev IM, Rylova AE, Sevast’yanov AI (1996) Formation of ozone and hydrogen peroxide during an electrical discharge in the solution-gas system. Russ J Electrochem 32:827–829
McClurkin JD, Maier DE, Ileleji KE (2013) Half-life time of ozone as a function of air movement and conditions in a sealed container. J Stored Prod Res 55:41–47
Kereszturi A, Gobi S (2014) Possibility of H2O2 decomposition in thin liquid films on Mars. Planet Space Sci 103:153–166
Cheng FC, Jen JF, Tasi TH (2002) Hydroxyl radical in living systems and its separation methods. J Chromatogr B 781:481–496
Breusegem FV, Vranová E, Dat JF, Inzé D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Ameta SC, Punjabi PB, Chobisa CS, Mangal N, Bhardwaj R (1990) Singlet molecular oxygen. Asian J Chem Rev 1:106–124
Lei LC, Zhang Y, Zhang XW, Du YX, Dai QZ, Han S (2007) Degradation performance of 4-Chlorophenol as a typical organic pollutant by a pulsed high voltage discharge system. Ind Eng Chem Res 46:5469–5477
Wang H, Li J, Quan X (2006) Decoloration of azo dye by a multi-needle-to-plate high-voltage pulsed corona discharge system in water. J Electrostat 64:416–421
Yin X, Bian W, Shi J (2009) 4-chlorophenol degradation by pulsed high voltage discharge coupling internal electrolysis. J Hazard Mater 166:1474–1479
Bian W, Song X, Liu D, Zhang J, Chen X (2013) Actions of nitrogen plasma in the 4-chrolophenol degradation by pulsed high-voltage discharge with bubbling gas. Chem Eng J 219:385–394
Bian W, Song X, Liu D, Zhang J, Chen X (2011) The intermediate products in the degradation of 4-chlorophenol by pulsed high voltage discharge in water. J Hazard Mater 192:1330–1339
Bian W, Ying X, Shi J (2009) Enhance degradation of p-chlorophenol in a novel pulsed high voltage discharge reactor. J Hazard Mater 162:906–912
Bian W, Song X, Shi J, Yin X (2012) Nitrogen fixed into HNO3 by pulsed high voltage discharge. J Electrostat 70:317–326
Sun B, Aye NN, Gao Z, Lv D, Zhu X, Sato M (2012) Characteristics of gas-liquid pulsed discharge plasma reactor and dye decoloration efficiency. J Environ Sci 24:840–845
Zhang J, Liu D, Bian W, Chen X (2012) Degradation of 2,4-dichorophenol by pulsed high voltage discharge in water. Desalination 304:49–56
Tsea KL, Martinb T, McFarlanea CM, Nienowa AW (2003) Small bubble formation via a coalescence dependent break-up mechanism. Chem Eng Sci 58:275–286
Jo D, Revankar ST (2010) Effect of coalescence and breakup on bubble size distributions in a two-dimensional packed bed. Chem Eng Sci 65:4231–4238
Busciglio A, Grisafi F, Scargiali F, Brucato A (2010) On the measurement of bubble size distribution in gas-liquid contactors via light sheet and image analysis. Chem Eng Sci 65:2558–2568
Wongsuchoto P, Charinpanitkul T, Pavasant P (2003) Bubble size distribution and gas-liquid mass transfer in airlift contactors. Chem Eng J 92:81–90
Majumder SK, Kundu G, Mukherjee D (2006) Bubble size distribution and gas-liquid interfacial area in a modified down flow bubble column. Chem Eng J 122:1–10
Sotiriadis AA, Thorpe RB, Smith JM (2005) Bubble size and mass transfer characteristics of sparged downwards two-phase flow. Chem Eng Sci 60:5917–5929
Danckwerts PV (1951) Singnificance of liquid-film coefficients in gas absorption. Ind Eng Chem 43:1460–1467
DeCoursey WJ (1974) Absorption with chemical reaction: development of a new relation for the Danckwerts model. Chem Eng Sci 29:1867–1872
Khan W (1990) An extension of Danckwerts theoretical surface renewal model to mass transfer at contaminated turbulent interface. Math Comput Model 14:750–754
Zhao W, Shi H, Wang D (2004) Modeling of mass transfer characteristics of bubble column reactor with surfactant present. J Zhejiang Univ Sci 5:714–720
Linek V, Moucha T, Kordač M (2005) Mechanism of mass transfer from bubbles in dispersions Part I. Danckwerts’ plot method with sulphite solutions in the presence of viscosity and surface tension changing agents. Chem Eng Process 44:353–361
Joshi JB (2001) Computation flow modeling and design of bubble column reactors. Chem Eng Sci 56:5893–5933
Painmanakul P, Loubière K, Hébrard G, Mietton-Peuchot M, Roustan M (2005) Effect of surfactants on liquid-side mass transfer coefficients. Chem Eng Sci 60:6480–6491
Sardeing P, Painmanakul P, Hébrard G (2006) Effect of surfactants on liquid-side mass transfer coefficients in gas-liquid systems: a first step to modeling. Chem Eng Sci 61:6249–6260
Vázquez G, Cancela MA, Riverol C, Alvarez E, Navaza JM (2000) Application of Danckwerts method in a bubble column Effects of surfactants on mass transfer coefficient and interfacial area. Chem Eng J 78:13–19
Cents AHG, De Bruijn FT, Brilman DWF, Versteeg GF (2005) Validation of the Danckwerts-plot technique by simultaneous chemical absorption of CO2 and physical desorption of O2. Chem Eng Sci 60:5809–5818
Danckwerts PV, Kennedy AM, Roberts D (1963) Kinetics of CO2 absorption in alkaline solutions-II: absorption in a packed column and tests of surface-renewal models. Chem Eng Sci 18:63–72
Roberts D, Danckwerts PV (1962) Kinetics of CO2 absorption in alkaline solutions-I: transient absorption rates and catalysis by arsenite. Chem Eng Sci 17:961–969
Dean DN, Fuchs MJ, Schaffer JM, Carbonell RG (1977) Batch absorption of CO2 by ree and microencapsulated carbonic anhydrase. Ind Eng Chem Fundam 16:452–458
Danckwerts PV, Arvo L (1970) Gas-liquid reactions. J Electrochem Soc 117:369C–370C
Cents AHG, Brilman DWF, Versteeg GF (2001) Gas absorption in an agitated gas–liquid–liquid system. Chem Eng Sci 56:1075–1083
Danckwerts PV, Sharma MM (1966) The absorption of carbon dioxide into solutions of alkalis and amines. Inst Chem Engrs 44:244–280
Richards GM, Ratcliff GA, Danckwerts PV (1964) Kinetics of CO2 absorption-III: first-order reaction in a packed column. Chem Eng Sci 19:325–328
Peng G, Liu J (2006) Improvement of hybird alkali measurement by adopting Chlorination barium method. J Jinling Inst Technol 22:102–105 (in Chinese)
Danckwerts PV, Gillham AJ (1966) The design of gas absorbers, I-methods for predicting rates of absorption with chemical reaction in packed columns, and tests with 11/2 in. Raschig rings. Trans Inst Chem Engrs 44:T42
Perry RH, Green DW, Maloney JQ (1997) Perry’s chemical engineers’ handbook. Mc Graw-Hill, New York
Acknowledgments
The financial support of this work by the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Suzhou Key Laboratory of Green Chemical Engineering is gratefully acknowledged. We thank Professor Jian Zhu for the great help during the bubble image acquisition process.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, M., Zhao, K., Lu, Y. et al. An Integrated Approach to Understanding the Mass Transfer and Reaction Processes in a Pulsed High-Voltage Discharge Reactor. Plasma Chem Plasma Process 35, 721–738 (2015). https://doi.org/10.1007/s11090-015-9625-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9625-x