Abstract
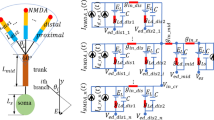
Evidence shows that distal dendritic polarization induced by electrical fields (EFs) can affect sublinear dendritic integration of AMPA synaptic inputs via modulating the amplitude of excitatory postsynaptic potential that is also influenced by subthreshold active hyperpolarization-active cation current (\(I_{\text{h}}\)). However, it remains unclear how \(I_{\text{h}}\) participates in EF-regulated dendritic integration and then affects the neural input/output relationship. To this end, a two-compartment model was established to depict the effect of \(I_{\text{h}}\) on EF-regulated sublinear dendritic integration as well as its influence on the initiation of action potentials. With the singular perturbation method we found that the equilibrium mapping of the fast subsystem can serve as the asymptotic subthreshold input/output function for EF-regulated sublinear dendritic integration in the presence of \(I_{\text{h}}\). Both theoretical and simulation results showed that the EF-regulated sublinear dendritic integration in the presence of \(I_{\text{h}}\), depending on the biophysical properties of \(I_{\text{h}}\) including conductance and steady-state activation function, becomes more pronounced sublinear for anodal EF stimulation while is more linear for cathodal stimulation. Further, the presence of \(I_{\text{h}}\), independent of EF polarities, reduces the sensitivity of EF modulation effect on dendritic integration via diminishing EF-induced dendritic polarizations. By identifying the respective contribution of EF-regulated dendritic integration and EF-induced somatic polarization to an action potential generation in the presence of \(I_{\text{h}}\), we found that \(I_{\text{h}}\) inhibits the initiation of action potential no matter of EF polarities, which is, respectively, attributed to the \(I_{\text{h}}\) activation-induced more pronounced sublinear dendritic integration in the presence of anodal EF and the \(I_{\text{h}}\) inactivation-induced larger somatic hyperpolarization in the case of cathodal EF. These findings suggest that the specific modulatory influence of EF on brain activities not only depends on the EF parameters but also relies on the neural intrinsic properties.








Similar content being viewed by others
References
Brunoni, A.R., Moffa, A.H., Sampaio-Junior, B., Borrione, L., et al.: Trial of electrical direct-current therapy versus escitalopram for depression. N. Engl. J. Med. 376(26), 2523–2533 (2017). https://doi.org/10.1056/NEJMoa1612999
Szymkowicz, S.M., McLaren, M.E., Suryadevara, U., Woods, A.J.: Transcranial direct current stimulation use in the treatment of neuropsychiatric disorders: a brief review. Psychiatr. Ann. 46(11), 642–646 (2016). https://doi.org/10.3928/00485713-20161006-01
Wagner, T., Valero-Cabre, A., Pascual-Leone, A.: Noninvasive human brain stimulation. Annu. Rev. Biomed. Eng. 9, 527–565 (2007). https://doi.org/10.1146/annurev.bioeng.9.061206.133100
Flöel, A.: tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 85(Pt 3), 934–947 (2013). https://doi.org/10.1016/j.neuroimage.2013.05.098
Schlaug, G., Renga, V., Nair, D.: Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 65(12), 1571–1576 (2008). https://doi.org/10.1001/archneur.65.12.1571
Conforto, A.B., Servinsckins, L., de Paiva, J.P.Q., Amaro, E., et al.: Safety of cathodal transcranial direct current stimulation early after ischemic stroke. Brain Stimul. 12(2), 374–376 (2018). https://doi.org/10.1016/j.brs.2018.11.009
Jackson, M.P., Rahman, A., Lafon, B., Kronberg, G., et al.: Animal models of transcranial direct current stimulation: methods and mechanisms. Clin. Neurophysiol. 127(11), 3425–3454 (2016). https://doi.org/10.1016/j.clinph.2016.08.016
Bikson, M., Inoue, M., Akiyama, H., Deans, J.K., et al.: Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 557(Pt. 1), 175–190 (2004). https://doi.org/10.1113/jphysiol.2003.055772
Radman, T., Ramos, R.L., Brumberg, J.C., Bikson, M.: Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2(4), 215–228 (2009). https://doi.org/10.1016/j.brs.2009.03.007
Reato, D., Rahman, A., Bikson, M., Parra, L.C.: Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 30(45), 15067–15079 (2010). https://doi.org/10.1523/jneurosci.2059-10.2010
Kronberg, G., Bridi, M., Abel, T., Bikson, M., Parra, L.C.: Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 10(1), 51–58 (2016). https://doi.org/10.1016/j.brs.2016.10.001
Yi, G.S., Wei, X.L., Wang, J., Deng, B., Che, Y.Q.: Modulations of dendritic Ca2+ spike with weak electric fields in layer 5 pyramidal cells. Neural Netw. 110, 8–18 (2019). https://doi.org/10.1016/j.neunet.2018.10.013
Radman, T., Su, Y., An, J.H., Bikson, M.: Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J. Neurosci. 27(11), 3030–3036 (2007). https://doi.org/10.1523/jneurosci.0095-07.2007
Rahman, A., Lafon, B., Parra, L.C., Bikson, M.: Direct current stimulation boosts synaptic gain and cooperativity in vitro. J. Physiol. 595(11), 3535–3547 (2017). https://doi.org/10.1113/jp273005
Lafon, B., Rahman, A., Bikson, M., Parra, L.C.: Direct current stimulation alters neuronal input/output function. Brain Stimul. 10(1), 36–45 (2017). https://doi.org/10.1016/j.brs.2016.08.014
Fan, Y.Q., Wei, X.L., Yi, G.S., Lu, M.L., et al.: Asymptotic input-output relationship predicts electric field effect on sublinear dendritic integration of AMPA synapses. Neural Comput. 33, 1–37 (2021). https://doi.org/10.1162/neco_a_01438
Tran-Van-Minh, A., Cazé, R.D., Abrahamsson, T., Cathala, L., et al.: Contribution of sublinear and supralinear dendritic integration to neuronal computations. Front. Cell. Neurosci. 9, 67 (2015). https://doi.org/10.3389/fncel.2015.00067
Kole, M.H.P., Hallermann, S., Stuart, G.J.: Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J. Neurosci. 26(6), 1677–1687 (2006). https://doi.org/10.1523/jneurosci.3664-05.2006
Harnett, M.T., Magee, J.C., Williams, S.R.: Distribution and function of HCN channels in the apical dendritic tuft of neocortical pyramidal neurons. J. Neurosci. 35(3), 1024–1037 (2015). https://doi.org/10.1523/jneurosci.2813-14.2015
Magee, J.C.: Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. 18(19), 7613–7624 (1998). https://doi.org/10.1523/jneurosci.18-19-07613.1998
Williams, S.R., Stuart, G.J.: Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J. Neurophysiol. 83(5), 3177–3182 (2000). https://doi.org/10.1152/jn.2000.83.5.3177
George, M.S., Abbott, L.F., Siegelbaum, S.A.: HCN hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K(+) channels. Nat. Neurosci. 12, 577–584 (2009). https://doi.org/10.1038/nn.2307
Migliore, M., Miglore, R.: Know your current I(h): interaction with a shunting current explains the puzzling effects of its pharmacological or pathological modulations. PLoS ONE 7(5), e36867 (2012). https://doi.org/10.1371/journal.pone.0036867
Toloza, E.H.S., Negahbani, E., Fröhlich, F.: Ih interacts with somato-dendritic structure to determine frequency response to weak alternating electric field stimulation. J. Neurophysiol. 119(3), 1029–1036 (2017). https://doi.org/10.1152/jn.00541.2017
Hao, X.M., Xu, R., Chen, A.Q., Sun, F.J., et al.: Endogenous HCN channels modulate the firing activity of globus pallidus neurons in parkinsonian animals. Front. Aging Neurosc. 11, 190 (2019). https://doi.org/10.3389/fnagi.2019.00190
Yi, G.S., Wang, J., Wei, X.L., Tsang, K.M.: Exploring how extracellular electric field modulates neuron activity through dynamical analysis of a two-compartment neuron model. J. Comput. Neurosci. 36(3), 383–399 (2014). https://doi.org/10.1007/s10827-013-0479-z
Park, E.H., So, P., Barreto, E., Gluckman, B.J., Schiff, S.J.: Electric field modulation of synchronization in neuronal networks. Neurocomputing 52–54, 169–175 (2003). https://doi.org/10.1016/S0925-2312(02)00820-2
Park, E.H., Barreto, E., Gluckman, B.J., Schiff, S.J., So, P.: A model of the effects of applied electric fields on neuronal synchronization. J. Comput. Neurosci. 19(1), 53–70 (2005). https://doi.org/10.1007/s10827-005-0214-5
Sarid, L., Feldmeyer, D., Gidon, A., Sakmann, B., Segev, I.: Contribution of intracolumnar layer 2/3-to-layer 2/3 excitatory connections in shaping the response to whisker deflection in rat barrel cortex. Cereb. Cortex 25, 849–858 (2013). https://doi.org/10.1093/cercor/bht268
Yi, G.S., Wang, J., Deng, B., Wei, X.L.: Morphology controls how hippocampal CA1 pyramidal neuron responds to uniform electric fields: a biophysical modeling study. Sci. Rep. 7(1), 3210 (2017). https://doi.org/10.1038/s41598-017-03547-6
Cavarretta, F., Carnevale, N.T., Tegolo, D., Migliore, M.: Effects of low frequency electric fields on synaptic integration in hippocampal CA1 pyramidal neurons: Implications for power line emissions. Front. Cell. Neurosci. 8, 310 (2014). https://doi.org/10.3389/fncel.2014.00310
Larkum, M.E., Nevian, T., Sandler, M., Polsky, A., Schiller, J.: Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325(5941), 756–760 (2009). https://doi.org/10.1126/science.1171958
Berzhanskaya, J., Chernyy, N., Gluckman, B.J., Schiff, S.J., Ascoli, G.A.: Modulation of hippocampal rhythms by subthreshold electric fields and network topology. J. Comput. Neurosci. 34(3), 369–389 (2013). https://doi.org/10.1007/s10827-012-0426-4
Hines, M.L., Carnevale, N.T.: The NEURON simulation environment. Neural Comput. 9(6), 1179–1209 (1997)
Poleg-Polsky, A.: Effects of neural morphology and input distribution on synaptic processing by global and focal NMDA-spikes. PLoS ONE 10(10), e0140254 (2015). https://doi.org/10.1371/journal.pone.0140254
MeyerBase, A., Ohl, F., Scheich, H.: Singular perturbation analysis of competitive neural networks with different time scales. Neural Comput. 8(8), 1731–1742 (1996). https://doi.org/10.1162/neco.1996.8.8.1731
Kuznetsov, Y.A., Muratori, S., Rinaldi, S.: Homoclinic bifurcations in slow-fast second order systems. Nonlinear Anal. Theory Methods Appl. 25(7), 747–762 (1995). https://doi.org/10.1016/0362-546X(94)E0005-2
Longordo, F., To, M.S., Ikeda, K., Stuart, G.J.: Sublinear integration underlies binocular processing in primary visual cortex. Nat. Neurosci. 16(6), 714–723 (2013). https://doi.org/10.1038/nn.3394
Ceballos, C.C., Pena, R.F.O., Roque, A.C.: Impact of the activation rate of the hyperpolarization-activated current I-h on the neuronal membrane time constant and synaptic potential duration. The Eur. Phys. J. Special Top. (2021). https://doi.org/10.1140/epjs/s11734-021-00176-z
Berger, T., Larkum, M.E., Luscher, H.R.: High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J. Neurophysiol. 85(2), 855–868 (2001). https://doi.org/10.1152/jn.2001.85.2.855
Cazé, R.D., Humphries, M., Gutkin, B.: Passive dendrites enable single neurons to compute linearly non-separable functions. Plos Comput. Biol. 9(2), e1002867 (2013). https://doi.org/10.1371/journal.pcbi.1002867
Tsay, D., Dudman, J.T., Siegelbaum, S.A.: HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron 56(6), 1076–1089 (2007). https://doi.org/10.1016/j.neuron.2007.11.015
Nolan, M.F., Malleret, G., Dudman, J.T., Buhl, D.L., et al.: A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119, 719–732 (2004). https://doi.org/10.1016/s0092-8674(04)01055-4
Santoro, B., Lee, J.Y., Englot, D.J., Gildersleeve, S., et al.: Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia 51(8), 1624–1627 (2010). https://doi.org/10.1111/j.1528-1167.2010.02554.x
Giocomo, L.M., Hussaini, S.A., Zheng, F., Kandel, E.R., et al.: Grid cells use HCN1 channels for spatial scaling. Cell 147, 1159–1170 (2011). https://doi.org/10.1016/j.cell.2011.08.051
Hussaini, S.A., Kempadoo, K.A., Thuault, S.J., Siegelbaum, S.A., Kandel, E.R.: Increased size and stability of CA1 and CA3 place fields in HCN1 knockout mice. Neuron 72, 643–653 (2011). https://doi.org/10.1016/j.neuron.2011.09.007
Tranchina, D., Nicholson, C.: A model for the polarization of neurons by extrinsically applied electric fields. Biophys. J . 50(6), 1139–1156 (1986). https://doi.org/10.1016/s0006-3495(86)83558-5
Smith, S.L., Smith, I.T., Branco, T., Häusser, M.: Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503(7474), 115–120 (2013). https://doi.org/10.1038/nature12600
Gasparini, S., DiFrancesco, D.: Action of serotonin on the hyperpolarization-activated cation current (Ih) in rat CA1 hippocampal neurons. Eur. J. Neurosci. 11, 3093–3100 (1999)
Funding
This work was supported by the National Natural Science Foundation of China under Grant 62171312, 61771330, 62071324, and the Tianjin Municipal Natural Science Foundation under Grant 19JCQNJC01200.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YF. The first draft of the manuscript was written by YF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability
All data generated or used during this study are included in this published article and its supplementary information files.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11071_2022_7427_MOESM1_ESM.docx
Supplementary file1. In Sect. 1, we introduced the detailed parameters of STC-PYN-AMPA with S1 Table. In Sect. 2, we introduced the detailed parameters of active ionic currents of a biophysical layer 5 pyramidal neuron with S2 Table. In Sect. 3, we introduced the detailed parameters of multi current clamp stimulation used for repolarizing resting potential as changes with S3 Table. (DOCX 99 KB)
11071_2022_7427_MOESM2_ESM.docx
Supplementary file2. In Sect. 1, we showed the effect of on EF-regulated dendritic integration of NMDA synapses with the layer 5 pyramidal neuron introduced in Materials and Methods, as shown in S1 Fig. In Sect. 2, we studied the effect of on EF-regulated sublinear dendritic integration with a biophysical pyramidal model in CA1 that has increasing from soma to distal dendrites, as shown in S2 Fig. (DOCX 153 KB)
Rights and permissions
About this article
Cite this article
Fan, Y., Wei, X., Yi, G. et al. Effects of hyperpolarization-active cation current (Ih) on sublinear dendritic integration under applied electric fields. Nonlinear Dyn 108, 4335–4356 (2022). https://doi.org/10.1007/s11071-022-07427-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-022-07427-1