Abstract
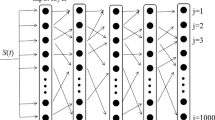
The feedforward neural network is widely applied in various machine learning architectures, in which the synaptic weight between layers plays an important role in the weak signal propagation. In this paper, the five-layer Izhikevich neural networks with excitatory or excitatory–inhibition neurons are employed to study the effect of Gaussian white noise and synaptic weight between layers on the weak signal transmission characteristics of the subthreshold excitatory postsynaptic currents signal imposed on the input layer. It can be found that there is an optimum value of noise intensity (a medium noise intensity) at which the weak signal can be stably propagated in the five-layer Izhikevich neural networks. The spike timing precision (STP) under the optimal noise intensity will become maximum by increasing the synaptic weight. The noise intensity and synaptic weight corresponding to the maximum value of the STP in the excitatory–inhibition network are smaller than those in excitatory–inhibition network. For the smaller or the bigger noise intensity, however, the STP will become very small, and the weak signal cannot be transmitted from the input layer to the output layer. Furthermore, the weak signal is propagated from the input layer to the output layer and enhanced under a larger synaptic weight in the feedforward neural networks.












Similar content being viewed by others
References
Kumar, A., Rotter, S., Aertsen, A.: Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nat. Rev. Neurosci. 11, 615–627 (2010)
Guo, D.Q., Li, C.G.: Signal propagation in feedforward neuronal networks with unreliable synapses. J. Comput. Neurosci. 30, 567–587 (2011)
Litvak, V., Sompolinsky, H., Segev, I., Abeles, M.: On the transmission of rate code in long feedforward networks with excitatory–inhibitory balance. J. Neurosci. 23, 3006–3015 (2003)
Reyes, A.D.: Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nat. Neurosci. 6, 593–599 (2003)
Feinerman, O., Segal, M., Moses, E.: Signal propagation along unidimensional neuronal networks. J. Neurophysiol. 94, 3406–3416 (2005)
Feinerman, O., Moses, E.: Transport of information along unidimensional layered networks of dissociated hippocampal neurons and implications for rate coding. J. Neurosci. 26, 4526–4534 (2006)
August, D.A., Levy, W.B.: Temporal sequence compression by an integrate-and-fire model of hippocampal area CA3. J. Comput. Neurosci. 6, 71–90 (1999)
Lee, A.K., Wilson, M.A.: Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 (2002)
Qin, Y.M., Wang, J., Men, C., Deng, B., Wei, X.L.: Vibrational resonance in feedforward network. Chaos 21, 023133 (2011)
Men, C., Wang, J., Qin, Y.M., Deng, B., Tsang, K.M., Chan, W.L.: Propagation of spiking regularity and double coherence resonance in feedforward networks. Chaos 22, 013104 (2012)
Gao, Y., Wang, J.: Oscillation propagation in neural networks with different topologies. Phys. Rev. E 83, 031909 (2011)
Faisal, A.A., Selen, L.P.J., Wolpert, D.M.: Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303 (2008)
Softky, W., Koch, C.: The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 13, 334–350 (1993)
Qin, Y., Wang, J., Men, C., Deng, B., Wei, X., Yu, H.: The effects of time delay on the stochastic resonance in feed-forward-loop neuronal network motifs. Commun. Nonlinear Sci. 19(4), 3660–3670 (2014)
Collins, J.J., Chow, C.C., Imhoff, T.T.: Stochastic resonance without tuning. Nature 376, 236–238 (1995)
Eichwald, C., Walleczek, J.: Aperiodic stochastic resonance with chaotic input signals in excitable systems. Phys. Rev. E 55, 6315–6318 (1997)
Perc, M.: Noise-induced spatial periodicity in excitable chemical media. Chem. Phys. Lett. 410, 49–53 (2005)
Perc, M.: Stochastic resonance on excitable small-world networks via a pacemaker. Phys. Rev. E 76, 066203 (2007)
Parmananda, P., Santos, G., Escalera, J., Rivera, M., Showalter, K.: Stochastic resonance of electrochemical aperiodic spike trains. Phys. Rev. E 71, 031110 (2005)
Yilmaz, E., Baysal, V., Perc, M., Ozer, M.: Enhancement of pacemaker induced stochastic resonance by an autapse in a scale-free neuronal network. Sci. China Technol. Sci. 59, 364–370 (2016)
Perc, M.: Stochastic resonance on excitable small-world networks via a pacemaker. Phys. Rev. E 76(2), 066203 (2007)
Yilmaz, E., Uzuntarla, M., Ozer, M., Perc, M.: Stochastic resonance in hybrid scale-free neuronal networks. Phys. A 392(22), 5735–5741 (2013)
Hornic, K.: Multilayer feedforward networks are universal approximators. Neural Netw. 2, 359–366 (1989)
Ozer, M., Perc, M., Uzuntarla, M., Koklukaya, E.: Weak signal propagation through noisy feedforward neuronal networks. Neuroreport 21, 338–43 (2010)
Jeyakumari, S., Chinnathambi, V., Rajasekar, S., et al.: Single and multiple vibrational resonance in a quintic oscillator with monostable potentials. Phys. Rev. E 80(2), 046608 (2009)
Yilmaz, E., Baysal, V., Ozer, M., Perc, M.: Autaptic pacemaker mediated propagation of weak rhythmic, activity across small-world neuronal networks. Phys. A 444, 538–546 (2016)
Ullner, E., Zaikin, A., Garcıa-Ojalvo, J., Bascones, R., Kurths, J.: Vibrational resonance and vibrational propagation in excitable systems. Phys. Let. A 312(5), 348–354 (2003)
Deng, B., Wang, J., Wei, X.: Effect of chemical synapse on vibrational resonance in coupled neurons. Chaos 19(1), 013117 (2009)
Eichwald, C., Walleczek, J.: Aperiodic stochastic resonance with chaotic input signals in excitable systems. Phys. Rev. E. 55, 6315–6318 (1997)
Wang, H.T., Chen, Y.: Response of autaptic Hodgkin–Huxley neuron with noise to subthreshold sinusoidal signals. Phys. A 462, 321–329 (2016)
Sun, X.J., Liu, Z.F.: Combined effects of time delay and noise on the ability of neuronal network to detect the subthreshold signal. Nonlinear Dyn. 92(4), 1707–1717 (2018)
Yao, Y.G., Yang, L.J., Wang, C.J., Liu, Q., Gui, R., Xiong, J., et al.: Subthreshold periodic signal detection by bounded noise-induced resonance in the FitzHugh–Nagumo neuron. Complexity 2018, 5632650 (2018)
Arredondo, L.T., Perez, C.A.: Spatially coincident vibrotactile noise improves subthreshold stimulus detection. PLoS ONE 12, e0186932 (2017)
Whittington, M.A., Jefferys, J.G.R.: Recurrent excitatory postsynaptic potentials induced by synchronized fast cortical oscillations. Proc. Natl. Acad. Sci. 94, 12198–12203 (1997)
Xue, M., Atallah, B.V., Scanziani, M.: Equalizing excitation–inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014)
Clark, K.A., Collingridge, G.L.: Synaptic potentiation of dual-component excitatory postsynaptic currents in the rat hippocampus. J. Physiol. 482, 39–52 (1995)
Izhikevich, E.M.: Simple model of spiking neurons. IEEE Trans. Neural Netw. 4(6), 1569–1572 (2003)
Ren, G., Xue, Y., Lia, Y., Ma, J.: Field coupling benefits signal exchange between Colpitts systems. Appl. Math. Comput. 342, 45–54 (2019)
Guo, D.Q., Li, C.G.: Stochastic and coherence resonance in feedforward-loop neuronal network motifs. Phy. Rev. E 79(5), 051921 (2009)
Jiang, X., Liu, C., Wang, J.: Delay-induced multiple vibrational resonance in the bi-fan neuronal network motifs. BMEI 7, 288–296 (2014)
Ge, M., Jia, Y., Kirunda, J.B., Xu, Y., Shen, J., Lu, L., et al.: Propagation of firing rate by synchronization in a feed-forward multilayer Hindmarsh–Rose neural network. Neurocomputing 320, 60–68 (2018)
Ge, M., Xu, Y., Zhang, Z., et al.: Autaptic modulation-induced neuronal electrical activities and wave propagation on network under electromagnetic induction. Eur. Phys. J. Spec. Top. 227, 799–809 (2018)
Lu, L., Jia, Y., Kirunda, J.B., et al.: Effects of noise and synaptic weight on propagation of subthreshold excitatory postsynaptic current signal in a feed-forward neural network. Nonlinear Dyn. 95, 1673–1686 (2019)
Hansel, D., Mato, G., Meunier, C.: Phase dynamics for weakly coupled Hodgkin–Huxley neurons. EPL 23(5), 367–372 (1993)
Destexhe, A., Mainen, Z.F., Sejnowski, T.J.: Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. J. Comput. Neurosci. 1, 195–230 (1994)
Yang, L., Jia, Y., Yi, M.: The effects of electrical coupling on the temporal coding of neural signal in noisy Hodgkin-Huxley neuron ensemble. In: Sixth international conference on natural computation, vol 2, pp 819–823 (2010)
Pei, X., Wilkens, L., Moss, F.: Noise-mediated spike timing precision from aperiodic stimuli in an array of Hodgekin–Huxley-type neurons. Phys. Rev. Lett. 77, 4679 (1996)
Wang, S., Wang, W., Liu, F.: Propagation of firing rate in a feed-forward neuronal network. Phys. Rev. Let. 96, 018103 (2006)
Lu, L., Jia, Y., Xu, Y., Ge, M., Yang, L., Zhan, X.: Energy dependence on modes of electric activities of neuron driven by different external mixed signals under electromagnetic induction. Sci. China Technol. Sci. 62, 427–440 (2019)
Xu, Y., Jia, Y., Ma, J., Hayat, T., Alsaedi, A.: Collective responses in electrical activities of neurons under field coupling. Sci. Rep. 8, 1349 (2018)
Ge, M., Jia, Y., Xu, Y., Yang, L.: Mode transition in electrical activities of neuron driven by high and low frequency stimulus in the presence of electromagnetic induction and radiation. Nonlinear Dyn. 91(1), 515–523 (2018)
Ge, M., Jia, Y., Xu, Y., Lu, L., Wang, H., Zhao, Y.: Wave propagation and synchronization induced by chemical autapse in chain feed-forward Hindmarsh–Rose neural network. Appl. Math. Comput. 352, 136–145 (2019)
Rostamia, Z., Pham, V.-T., Jafari, S., Hadaeghic, F., Ma, J.: Taking control of initiated propagating wave in a neuronal network using magnetic radiation. Appl. Math. Comput. 338, 141–151 (2018)
Ma, J., Zhang, G., Hayat, T., Ren, G.: Model electrical activity of neuron under electric field. Nonlinear Dyn. 95, 1585–1598 (2019)
Lv, M., Wang, C.N., Ren, G.D., Ma, J.: Model of electrical activity in a neuron under magnetic flow effect. Nonlinear Dyn. 85, 1479–1490 (2016)
Xu, Y., Ma, J., Zhan, X., Yang, L., Jia, Y.: Temperature effect on memristive ion channels. Cogn. Neurodyn. (2019). https://doi.org/10.1007/s11571-019-09547-8
Liu, Y., Ma, J., Xu, Y., Jia, Y.: Electrical mode transition of hybrid neuronal model induced by external stimulus and electromagnetic induction. Int. J. Bifurc. Chaos 29, 1950156 (2019)
Yilmaz, E., Ozer, M., Baysal, V., Perc, M.: Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 6, 30914 (2016)
Xu, Y., Ying, H., Jia, Y., Ma, J., Hayat, T.: Autaptic regulation of electrical activities in neuron under electromagnetic induction. Sci. Rep. 7, 43452 (2017)
Yao, Z., Ma, J., Yao, Y., Wang, C.: Synchronization realization between two nonlinear circuits via an induction coil coupling. Nonlinear Dyn. 96, 205–217 (2019)
Ma, J., Wu, F., Alsaedi, A., Tang, J.: Crack synchronization of chaotic circuits under field coupling. Nonlinear Dyn. 93, 2057–2069 (2018)
Ma, J., Tang, J.: A review for dynamics in neuron and neuronal network. Nonlinear Dyn. 89, 1569–1578 (2017)
Handa, H., Sharma, B.B.: Synchronization of a set of coupled chaotic FitzHugh–Nagumo and Hindmarsh–Rose neurons with external electrical stimulation. Nonlinear Dyn. 85, 1–16 (2016)
Baltanas, J.P., Caado, J.M.: Noise-induced resonances in the Hindmarsh–Rose neuronal model. Phys. Rev. E 65, 041915 (2002)
Xu, Y., Jia, Y., Kirunda, J.B., et al.: Dynamic behaviors in coupled neurons system with the excitatory and inhibitory autapse under electromagnetic induction. Complexity 2018, 3012743 (2018)
Xu, Y., Jia, Y., Wang, H., et al.: Spiking activities in chain neural network driven by channel noise with field coupling. Nonlinear Dyn. 95, 3237–3247 (2019)
Acknowledgements
This study was supported by the National Natural Science Foundation of China under Grant under Nos. 11775091 (Y.J.) and 11704140 (Y.Z.); the Natural Science Foundation of Hubei Province under No. 2017CFB116 (Y.Z.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, M., Jia, Y., Lu, L. et al. Propagation characteristics of weak signal in feedforward Izhikevich neural networks. Nonlinear Dyn 99, 2355–2367 (2020). https://doi.org/10.1007/s11071-019-05392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-019-05392-w