Abstract
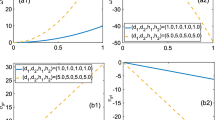
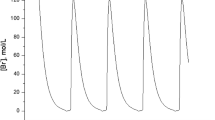
Experimental evidence has proved that calcium ions (\(\mathrm {Ca^{2+}}\)) play an important role in cellular physiological processes via calcium oscillations. The entry rate of \(\mathrm {Ca^{2+}}\) into cells through plasma membrane cells is a major modulator of intracellular \(\mathrm {Ca^{2+}}\) dynamics, including the voltage-gated \(\mathrm {Ca^{2+}}\) channel, the store-operated \(\mathrm {Ca^{2+}}\) channel (SOCC) and the receptor-operated \(\mathrm {Ca^{2+}}\) channel (ROCC). In this paper, we modify an established four-dimensional dynamical model, which contains the SOCC and ROCC, and carry out a bifurcation analysis to study dynamics of the model. In particular, Hopf bifurcation is identified with the maximum flow of the SOCC chosen as the bifurcation parameter, and normal form theory is applied to consider the stability of bifurcating limit cycles. Bifurcation of multiple limit cycles arising from generalized Hopf bifurcation is also discussed, which may yield complex dynamical behaviors. Further, it is shown that the variation of the maximum flows for different calcium channels determines the parameter range for stable oscillations, as well as for the frequency and amplitude of oscillations. The results indicate that Hopf bifurcation is the main source to generate oscillating behaviors, yielding a different bistable phenomenon which involves stable limit cycle and stable equilibrium. Moreover, it is shown that partially blocking the SOCC or the ROCC can change the parameter region of stable calcium oscillations, and the ROCC has more impact than the SOCC on amplitude or frequency of calcium oscillations.











Similar content being viewed by others
References
Clapham, D.E.: Calcium signaling. Cell 80(2), 259–268 (1995)
Ji, Q., Zhou, Y., Yang, Z., Meng, X.: Evaluation of bifurcation phenomena in a modified Shenc̈larter model for intracellular \({{\rm Ca}}^{2+}\) bursting oscillations. Nonlinear Dyn. 84(3), 1–8 (2016)
Gu, H., Pan, B.: A four-dimensional neuronal model to describe the complex nonlinear dynamics observed in the firing patterns of a sciatic nerve chronic constriction injury model. Nonlinear Dyn. 81(4), 2107–2126 (2015)
Pinto, M.C., Tonelli, F.M., Vieira, A.L., Kihara, A.H., Ulrich, H., Resende, R.R.: Studying complex system: calcium oscillations as attractor of cell differentiation. Integr. Biol. 8(2), 130–148 (2016)
Agulhon, C., Petravicz, J., McMullen, A.B., Sweger, E.J., Minton, S.K., Taves, S.R., Casper, K.B., Fiacco, T.A., McCarthy, K.D.: What is the role of astrocyte calcium in neurophysiology? Neuron 59(6), 932–946 (2008)
Pellerin, L., Magistretti, P.J.: Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 10(1), 53–62 (2004)
Fellin, T., Pascual, O., Haydon, P.G.: Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology 21(3), 208–215 (2006)
Navarrete, M., Perea, G., Maglio, L., Pastor, J., García de Sola, R., Araque, A.: Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb. Cortex 23(5), 1240–1246 (2012)
Cornell-Bell, A.H., Finkbeiner, S.M., Cooper, M.S., Smith, S.J.: Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247(4941), 470–473 (1990)
Hamilton, N.B., Attwell, D.: Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11(4), 227 (2010)
Parekh, A.B., Putney Jr., J.W.: Store-operated calcium channels. Physiol. Rev. 85(2), 757–810 (2005)
Putney Jr., J.W.: Presenilins, Alzheimer’s disease, and capacitative calcium entry. Neuron 27(3), 411–412 (2000)
Ong, H.L., Liu, X., Tsaneva-Atanasova, K., Singh, B.B., Bandyopadhyay, B.C., Swaim, W.D., Russell, J.T., Hegde, R.S., Sherman, A., Ambudkar, I.S.: Relocalization of STIM1 for activation of store-operated \({{{\rm Ca}}}^{2+}\) entry is determined by the depletion of subplasma membrane endoplasmic reticulum \({{{\rm Ca}}}^{2+}\) store. J. Biol. Chem. 282(16), 12176–12185 (2007)
Shuttleworth, T.J.: Stim and orai proteins and the non-capacitative ARC channels. Front. Biosci. 17, 847 (2012)
Ullah, G., Jung, P., Cornell-Bell, A.H.: Anti-phase calcium oscillations in astrocytes via inositol (1,4,5)-trisphosphate regeneration. Cell Calcium 39(3), 197–208 (2006)
Lopez-Caamal, F., Oyarzún, D.A., Middleton, R.H., García, M.R.: Spatial quantification of cytosolic \({{{\rm Ca}}}^{2+}\) accumulation in nonexcitable cells: an analytical study. IEEE/ACM Trans. Comput. Biol. Bioinf. (TCBB) 11(3), 592–603 (2014)
Riera, J., Hatanaka, R., Uchida, T., Ozaki, T., Kawashima, R.: Quantifying the uncertainty of spontaneous \({{{\rm Ca}}}^{2+}\) oscillations in astrocytes: particulars of Alzheimer’s disease. Biophys. J. 101(3), 554–564 (2011)
Di Garbo, A., Barbi, M., Chillemi, S., Alloisio, S., Nobile, M.: Calcium signalling in astrocytes and modulation of neural activity. Biosystems 89(1–3), 74–83 (2007)
Dupont, G., Lokenye, E.F.L., Challiss, R.J.: A model for \({{{\rm Ca}}}^{2+}\) oscillations stimulated by the type 5 metabotropic glutamate receptor: an unusual mechanism based on repetitive, reversible phosphorylation of the receptor. Biochimie 93(12), 2132–2138 (2011)
Postnov, D., Koreshkov, R., Brazhe, N., Brazhe, A., Sosnovtseva, O.: Dynamical patterns of calcium signaling in a functional model of neuron-astrocyte networks. J. Biol. Phys. 35(4), 425–445 (2009)
Li, Y.X., Rinzel, J.: Equations for \(\rm InsP_{3}\) receptor-mediated \({{{\rm Ca}}}^{2+}\) oscillations derived from a detailed kinetic model: a hodgkin-huxley like formalism. J. Theor. Biol. 166(4), 461–473 (1994)
Höfer, T., Venance, L., Giaume, C.: Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach. J. Neurosci. 22(12), 4850–4859 (2002)
Kummer, U., Olsen, L.F., Dixon, C.J., Green, A.K., Bornberg-Bauer, E., Baier, G.: Switching from simple to complex oscillations in calcium signaling. Biophys. J. 79(3), 1188–1195 (2000)
Dupont, G., Goldbeter, A.: One-pool model for \({{{\rm Ca}}}^{2+}\) oscillations involving \({{{\rm Ca}}}^{2+}\) and inositol 1,4,5-trisphosphate as co-agonists for \({{{\rm Ca}}}^{2+}\) release. Cell Calcium 14(4), 311–322 (1993)
Riera, J., Hatanaka, R., Ozaki, T., Kawashima, R.: Modeling the spontaneous \({{{\rm Ca}}}^{2+}\) oscillations in astrocytes: inconsistencies and usefulness. J. Integr. Neurosci. 10(04), 439–473 (2011)
Manninen, T., Havela, R., Linne, M.L.: Reproducibility and comparability of computational models for astrocyte calcium excitability. Front. Neuroinform. 11, 11 (2017)
Smyth, J.T., Hwang, S.Y., Tomita, T., DeHaven, W.I., Mercer, J.C., Putney, J.W.: Activation and regulation of store-operated calcium entry. J. Cell. Mol. Med. 14(10), 2337–2349 (2010)
Soboloff, J., Rothberg, B.S., Madesh, M., Gill, D.L.: Stim proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 13(9), 549 (2012)
Cao, P., Tan, X., Donovan, G., Sanderson, M.J., Sneyd, J.: A deterministic model predicts the properties of stochastic calcium oscillations in airway smooth muscle cells. PLoS Comput. Biol. 10(8), e1003783 (2014)
Hinrichsen, D., Pritchard, A.J.: Mathematical Systems Theory I: Modelling, State Space Analysis, Stability and Robustness, vol. 48. Springer, Berlin (2005)
Yu, P.: Closed-form conditions of bifurcation points for general differential equations. Int. J. Bifurc. Chaos 15(04), 1467–1483 (2005)
Yu, P.: Computation of normal forms via a perturbation technique. J. Sound Vib. 211(1), 19–38 (1998)
Zhang, W., Yu, P.: Hopf and generalized hopf bifurcations in a recurrent autoimmune disease model. Int. J. Bifurc. Chaos 26(05), 1650079 (2016)
Yu, P., Han, M.: Small limit cycles bifurcating from fine focus points in cubic order Z2-equivariant vector fields. Chaos Solitons Fractals 24(1), 329–348 (2005)
Putney, J.W., Broad, L.M., Braun, F.J., Lievremont, J.P., Bird, G.S.J.: Mechanisms of capacitative calcium entry. J. Cell Sci. 114(12), 2223–2229 (2001)
Malarkey, E.B., Ni, Y., Parpura, V.: \({{{\rm Ca}}}^{2+}\) entry through TRPC channels contributes to intracellular \({{{\rm Ca}}}^{2+}\) dynamics and consequent glutamate release from rat astrocytes. Glia 56(8), 821–835 (2008)
Jousset, H., Frieden, M., Demaurex, N.: Stim knockdown reveals that store-operated \({{{\rm Ca}}}^{2+}\) channels located close to sarco/endoplasmic \({{{\rm Ca}}}^{2+}\) ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J. Biol. Chem. 282(15), 11456–11464 (2007)
Croft, W., Reusch, K., Tilunaite, A., Russell, N.A., Thul, R., Bellamy, T.C.: Probabilistic encoding of stimulus strength in astrocyte global calcium signals. Glia 64(4), 537–552 (2016)
Berridge, M.J., Galione, A.: Cytosolic calcium oscillators. FASEB J. 2(15), 3074–3082 (1988)
Zhao, Z., Bing, J., Gu, H.: Bifurcations and enhancement of neuronal firing induced by negative feedback. Nonlinear Dyn. 86(3), 1–12 (2016)
Feudel, U., Neiman, A., Pei, X., Wojtenek, W., Braun, H., Huber, M., Moss, F.: Homoclinic bifurcation in a Hodgkin–Huxley model of thermally sensitive neurons. Chaos 10(1), 231–239 (2000)
Mandelblat, Y., Etzion, Y., Grossman, Y., Golomb, D.: Period doubling of calcium spike firing in a model of a Purkinje cell dendrite. J. Comput. Neurosci. 11(1), 43–62 (2001)
Acknowledgements
This work is partially supported by the Natural Sciences and Engineering Research Council of Canada (No. R2686A02). A. Zhou also thanks the supports received from the Tianjin University Ph.D. Training Program and Western University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, A., Liu, X. & Yu, P. Bifurcation analysis on the effect of store-operated and receptor-operated calcium channels for calcium oscillations in astrocytes. Nonlinear Dyn 97, 733–748 (2019). https://doi.org/10.1007/s11071-019-05009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-019-05009-2