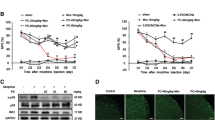
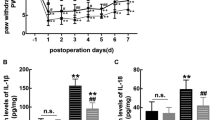
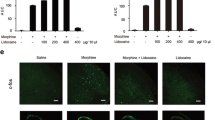
Abstract
Background and Purpose: Morphine is amongst the most effective analgesics available for the management of severe pain. However, prolonged morphine treatment leads to analgesic tolerance which limits its clinical usage. Previous studies have demonstrated that melatonin ameliorates morphine tolerance by reducing neuroinflammation. However, little is known about the relationship between Toll like receptor 2 (TLR2) and neuroinflammation in morphine tolerance. The aim of this study was to explore the role of TLR2 in morphine tolerance and its connections with melatonin and Nod-like receptor protein 3 (NLRP3) inflammasome. Methods: Sprague-Dawley rats were treated with morphine for 7 days and tail-flick latency test was performed to identify the induction of analgesic tolerance. The roles of TLR2 in microglia activation and morphine tolerance were assessed pharmacologically, and the possible interactions between melatonin, TLR2 and NLRP3 inflammasome were investigated. Key Results: Morphine tolerance was accompanied by increased TLR2 expression and NLRP3 inflammasome activation in spinal cord. whereas melatonin level was down-regulated. Chronic melatonin administration resulted in a reduced TLR2 expression and NLRP3 inflammasome activation. Moreover, the analgesic effect of morphine was partially restored. Inhibition of TLR2 suppressed the microglia and NLRP3 inflammasome activation, as well as restored the spinal melatonin level while attenuated the development of morphine tolerance. Furthermore, the inhibition of microglia activation ameliorated morphine tolerance via inhibiting TLR2-NLRP3 inflammasome signaling in spinal cord. Conclusion: In this study, we directly demonstrate a TLR2-melatonin negative feedback loop regulating microglia and NLRP3 inflammasome activation during the development of morphine tolerance.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fields HL (2011) The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69(4):591–594. https://doi.org/10.1016/j.neuron.2011.02.001
Abdel-Zaher AO, Mostafa MG, Farghaly HS, Hamdy MM, Abdel-Hady RH (2013) Role of oxidative stress and inducible nitric oxide synthase in morphine-induced tolerance and dependence in mice. Effect of alpha-lipoic acid. Behav Brain Res 247:17–26. https://doi.org/10.1016/j.bbr.2013.02.034
Kong H, Jiang CY, Hu L, Teng P, Zhang Y, Pan XX, Sun XD, Liu WT (2019) Morphine induces dysfunction of PINK1/Parkin-mediated mitophagy in spinal cord neurons implying involvement in antinociceptive tolerance. J Mol Cell Biol 11(12):1056–1068. https://doi.org/10.1093/jmcb/mjz002
Dang VC, Christie MJ (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165(6):1704–1716. https://doi.org/10.1111/j.1476-5381.2011.01482.x
Chao PK, Chang HF, Ou LC, Chuang JY, Lee PT, Chang WT, Chen SC, Ueng SH, Hsu JT, Tao PL, Law PY, Loh HH, Yeh SH (2019) Convallatoxin enhance the ligand-induced mu-opioid receptor endocytosis and attenuate morphine antinociceptive tolerance in mice. Sci Rep 9(1):2405. https://doi.org/10.1038/s41598-019-39555-x
Eidson LN, Murphy AZ (2019) Inflammatory mediators of opioid tolerance: implications for dependency and addiction. Peptides 115:51–58. https://doi.org/10.1016/j.peptides.2019.01.003
Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 24(33):7353–7365. https://doi.org/10.1523/JNEUROSCI.1850-04.2004
Zhang H, Li F, Li WW, Stary C, Clark JD, Xu S, Xiong X (2016) The inflammasome as a target for pain therapy. Br J Anaesth 117(6):693–707. https://doi.org/10.1093/bja/aew376
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426. https://doi.org/10.1016/s1097-2765(02)00599-3
Kim JJ, Jo EK (2013) NLRP3 inflammasome and host protection against bacterial infection. J Korean Med Sci 28(10):1415–1423. https://doi.org/10.3346/jkms.2013.28.10.1415
Wang H, Huang M, Wang W, Zhang Y, Ma X, Luo L, Xu X, Xu L, Shi H, Xu Y, Wang A, Xu T (2021) Microglial TLR4-induced TAK1 phosphorylation and NLRP3 activation mediates neuroinflammation and contributes to chronic morphine-induced antinociceptive tolerance. Pharmacol Res 165:105482. https://doi.org/10.1016/j.phrs.2021.105482
Cai Y, Kong H, Pan YB, Jiang L, Pan XX, Hu L, Qian YN, Jiang CY, Liu WT (2016) Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J Neuroinflammation 13(1):53. https://doi.org/10.1186/s12974-016-0520-z
Zhang HM, Zhang Y (2014) Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res 57(2):131–146. https://doi.org/10.1111/jpi.12162
Zisapel N (2018) New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol 175(16):3190–3199. https://doi.org/10.1111/bph.14116
Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade. J Pineal Res 65(4):e12525. https://doi.org/10.1111/jpi.12525
Yuan H, Wu G, Zhai X, Lu B, Meng B, Chen J (2019) Melatonin and rapamycin attenuate Isoflurane-Induced Cognitive Impairment through Inhibition of Neuroinflammation by suppressing the mTOR Signaling in the Hippocampus of aged mice. Front Aging Neurosci 11:314. https://doi.org/10.3389/fnagi.2019.00314
Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S (2019) Melatonin attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front Immunol 10:1511. https://doi.org/10.3389/fimmu.2019.01511
Wu HM, Zhao CC, Xie QM, Xu J, Fei GH (2020) TLR2-Melatonin feedback Loop regulates the activation of NLRP3 inflammasome in Murine allergic airway inflammation. Front Immunol 11:172. https://doi.org/10.3389/fimmu.2020.00172
Schilling S, Chausse B, Dikmen HO, Almouhanna F, Hollnagel JO, Lewen A, Kann O (2021) TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner. Brain Behav Immun 96:80–91. https://doi.org/10.1016/j.bbi.2021.05.013
Gill R, Tsung A, Billiar T (2010) Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med 48(9):1121–1132. https://doi.org/10.1016/j.freeradbiomed.2010.01.006
Kopiasz L, Dziendzikowska K, Gajewska M, Oczkowski M, Majchrzak-Kuligowska K, Krolikowski T, Gromadzka-Ostrowska J (2021) Effects of Dietary Oat Beta-Glucans on Colon Apoptosis and Autophagy through TLRs and Dectin-1 Signaling Pathways-Crohn’s Disease Model Study. Nutrients 13(2). https://doi.org/10.3390/nu13020321
Milanesi S, Verzola D, Cappadona F, Bonino B, Murugavel A, Pontremoli R, Garibotto G, Viazzi F (2019) Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J Cell Physiol 234(7):10868–10876. https://doi.org/10.1002/jcp.27929
Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR (2015) Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 21(11):1326–1331. https://doi.org/10.1038/nm.3978
Lacagnina MJ, Watkins LR, Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol Ther 184:145–158. https://doi.org/10.1016/j.pharmthera.2017.10.006
Chen J, Wang G, Sun T, Ma C, Huo X, Kong Y (2021) Involvement of TCF7L2 in generation of morphine-induced antinociceptive tolerance and hyperalgesia by modulating TLR4/ NF-kappaB/NLRP3 in microglia. Toxicol Appl Pharmacol 416:115458. https://doi.org/10.1016/j.taap.2021.115458
Qu J, Tao XY, Teng P, Zhang Y, Guo CL, Hu L, Qian YN, Jiang CY, Liu WT (2017) Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. J Neuroinflammation 14(1):228. https://doi.org/10.1186/s12974-017-0997-0
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924. https://doi.org/10.4049/jimmunol.173.6.3916
Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, Ye B, Lian Q, Zhuo W, Si J, Chen S, Wang L (2021) Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunol Res 9(10):1111–1124. https://doi.org/10.1158/2326-6066.CIR-20-1019
Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22(1):114–123. https://doi.org/10.1016/j.bbi.2007.07.014
Lin SH, Huang YN, Kao JH, Tien LT, Tsai RY, Wong CS (2016) Melatonin reverses morphine tolerance by inhibiting microglia activation and HSP27 expression. Life Sci 152:38–43. https://doi.org/10.1016/j.lfs.2016.03.032
Lin F, Shan W, Zheng Y, Pan L, Zuo Z (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem 158(2):328–341. https://doi.org/10.1111/jnc.15368
Liu D, Zhou Y, Peng Y, Su P, Li Z, Xu Q, Tu Y, Tian X, Yang H, Wu Z, Mei W, Gao F (2018) Endoplasmic reticulum stress in spinal cord contributes to the development of Morphine Tolerance. Front Mol Neurosci 11:72. https://doi.org/10.3389/fnmol.2018.00072
Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J (2016) Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model, Neural Plast (2016) 5238730.https://doi.org/10.1155/2016/5238730
Yang H, Wu L, Deng H, Chen Y, Zhou H, Liu M, Wang S, Zheng L, Zhu L, Lv X (2020) Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-kappaB signaling pathway in spinal microglia. J Neuroinflammation 17(1):154. https://doi.org/10.1186/s12974-020-1731-x
Wang X, Tian S, Wang H, Liu P, Zheng H, Wu L, Liu Q, Wu W (2020) Botulinum toxin type a alleviates neuropathic pain and suppresses inflammatory cytokines release from microglia by targeting TLR2/MyD88 and SNAP23. Cell Biosci 10(1):141. https://doi.org/10.1186/s13578-020-00501-4
Zhang L, Meng J, Ban Y, Jalodia R, Chupikova I, Fernandez I, Brito N, Sharma U, Abreu MT, Ramakrishnan S, Roy S (2019) Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A 116(27):13523–13532. https://doi.org/10.1073/pnas.1901182116
Thomas JHL, Lui L, Abell A, Tieu W, Somogyi AA, Bajic JE, Hutchinson MR (2022) Toll-like receptors change morphine-induced antinociception, tolerance and dependence: studies using male and female TLR and signalling gene KO mice. Brain Behav Immun 102:71–85. https://doi.org/10.1016/j.bbi.2022.02.001
Kwilasz AJ, Todd LS, Duran-Malle JC, Schrama AEW, Mitten EH, Larson TA, Clements MA, Harris KM, Litwiler ST, Wang X, Van Dam AM, Maier SF, Rice KC, Watkins LR, Barrientos RM (2021) Experimental autoimmune encephalopathy (EAE)-induced hippocampal neuroinflammation and memory deficits are prevented with the non-opioid TLR2/TLR4 antagonist (+)-naltrexone. Behav Brain Res 396:112896. https://doi.org/10.1016/j.bbr.2020.112896
Wang L, Yang HY, Zang CX, Shang JM, Liu H, Zhang ZH, Yuan FY, Ju C, Li FY, Bao XQ, Zhang D (2021) TLR2 potentiates SR-Marco-Mediated neuroinflammation by interacting with the SRCR Domain. Mol Neurobiol 58(11):5743–5755. https://doi.org/10.1007/s12035-021-02463-1
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65(1):223–254. https://doi.org/10.1124/pr.112.005942
Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, Kielian T (2008) Microglia and astrocyte activation by toll-like receptor ligands: modulation by PPAR-gamma agonists. PPAR Res 2008:453120. https://doi.org/10.1155/2008/453120
Gong X, Chen Y, Chang J, Huang Y, Cai M, Zhang M (2018) Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J Neuroinflammation 15(1):262. https://doi.org/10.1186/s12974-018-1301-7
Inta D, Lang UE, Borgwardt S, Meyer-Lindenberg A, Gass P (2017) Microglia activation and Schizophrenia: Lessons from the Effects of Minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr Bull 43(3):493–496. https://doi.org/10.1093/schbul/sbw088
Liu Q, Su LY, Sun C, Jiao L, Miao Y, Xu M, Luo R, Zuo X, Zhou R, Zheng P, Xiong W, Xue T, Yao YG (2020) Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol 34:101560. https://doi.org/10.1016/j.redox.2020.101560
Derangula K, Javalgekar M, Kumar Arruri V, Gundu C, Kumar Kalvala A, Kumar A (2022) Probucol attenuates NF-kappaB/NLRP3 signalling and augments Nrf-2 mediated antioxidant defence in nerve injury induced neuropathic pain. Int Immunopharmacol 102:108397. https://doi.org/10.1016/j.intimp.2021.108397
Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM (2021) Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun 91:142–158. https://doi.org/10.1016/j.bbi.2020.09.017
Tian L, Yan J, Li K, Zhang W, Lin B, Lai W, Bian L, Liu H, Xi Z, Liu X (2021) Ozone exposure promotes pyroptosis in rat lungs via the TLR2/4-NF-kappaB-NLRP3 signaling pathway. Toxicology 450:152668. https://doi.org/10.1016/j.tox.2020.152668
Duffy EB, Periasamy S, Hunt D, Drake JR, Harton JA (2016) FcgammaR mediates TLR2- and syk-dependent NLRP3 inflammasome activation by inactivated Francisella tularensis LVS immune complexes. J Leukoc Biol 100(6):1335–1347. https://doi.org/10.1189/jlb.2A1215-555RR
Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR (2011) Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc 110(8):487–494. https://doi.org/10.1016/S0929-6646(11)60074-0
Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, Zagrean AM (2020) Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 10(9). https://doi.org/10.3390/biom10091211
Gonzalez-Gonzalez A, Garcia Nieto E, Gonzalez A, Sanchez-Fernandez C, Alonso-Gonzalez C, Menendez-Menendez J, Gomez-Arozamena J, Cos S (2019) Martinez-Campa, Melatonin Modulation of Radiation and Chemotherapeutics-induced changes on differentiation of breast fibroblasts. Int J Mol Sci 20(16). https://doi.org/10.3390/ijms20163935
Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L (2020) Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther 11(1):259. https://doi.org/10.1186/s13287-020-01756-x
Song L, Wu C, Zuo Y (2015) Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol 15:12. https://doi.org/10.1186/1471-2253-15-12
Luo J, Song J, Zhang H, Zhang F, Liu H, Li L, Zhang Z, Chen L, Zhang M, Lin D, Lin M, Zhou R (2018) Melatonin mediated Foxp3-downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. pylori infected mice. Int Immunopharmacol 64:116–122. https://doi.org/10.1016/j.intimp.2018.08.034
Funding
We would like to thank the National Natural Science Foundation of China (Grant no. 81974168) for their support in this research.
Author information
Authors and Affiliations
Contributions
Xiaoling Peng wrote the main manuscript text. Jihong Wang and Zheng Li prepared Figs. 1 and 2. Xiaoqian Jia, Anqi Zhang and Jie Ju prepared Figs. 3, 4 and 5. Volker Eulenburg and Feng Gao revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflict of financial interest or benefit. Informed consent was obtained from all individual participants included in the study.
Ethical Approval
The animal research was approved by the Animal Experimental Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Ethics Approval Number: TJH-202007007).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, X., Wang, J., Li, Z. et al. Toll-like Receptor 2-Melatonin Feedback Loop Regulates the Activation of Spinal NLRP3 Inflammasome in Morphine-Tolerant Rats. Neurochem Res 48, 3597–3609 (2023). https://doi.org/10.1007/s11064-023-03998-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03998-6