Abstract
COVID-19, initially regarded as specific lung disease, exhibits an extremely broad spectrum of symptoms. Extrapulmonary manifestations of the disease also include important neuropsychiatric symptoms with atypical characteristics. Are these disturbances linked to stress accompanying every systemic infection, or are due to specific neurobiological changes associated with COVID-19? Evidence accumulated so far indicates that the pathophysiology of COVID-19 is characterized by systemic inflammation, hypoxia resulting from respiratory failure, and neuroinflammation (either due to viral neurotropism or in response to cytokine storm), all affecting the brain. It is reasonable to hypothesize that all these events may initiate or worsen psychiatric and cognitive disorders. Damage to the brain triggers a specific type of reactive response mounted by neuroglia cells, in particular by astrocytes which are the homeostatic cell par excellence. Astrocytes undergo complex morphological, biochemical, and functional remodeling aimed at mobilizing the regenerative potential of the central nervous system. If the brain is not directly damaged, resolution of systemic pathology usually results in restoration of the physiological homeostatic status of neuroglial cells. The completeness and dynamics of this process in pathological conditions remain largely unknown. In a subset of patients, glial cells could fail to recover after infection thus promoting the onset and progression of COVID-19-related neuropsychiatric diseases. There is evidence from post-mortem examinations of the brains of COVID-19 patients of alterations in both astrocytes and microglia. In conclusion, COVID-19 activates a huge reactive response of glial cells, that physiologically act as the main controller of the inflammatory, protective and regenerative events. However, in some patients the restoration of glial physiological state does not occur, thus compromising glial function and ultimately resulting in homeostatic failure underlying a set of specific neuropsychiatric symptoms related to COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Role of Astrocytes in the Brain: May the Homeostatic Cells Par Excellence Become SARS CoV-2 Damage Effectors?
Astroglia control such a huge number of functions that they can be considered to take part in any circumstance in which there is a disturbance of cerebral homeostasis. In fact, following any brain insults, these cells become reactive by profoundly modifying their morphology and functions. This complex response is part of the homeostatic tasks that glial cells perform physiologically and has as its objective the containment of the damage and the return to a homeostatic condition. However, glia changes are not always restored in a timely manner thus causing brain damage. At the beginning of 2021, we hypothesized that the neuropsychiatric consequences of COVID-19 are maladaptive glial recovery to blame [1, 2]. This hypothesis is now reinforced by growing evidence which is summarized in the following paragraphs.
Glial cells are heterogeneous neural cells exerting a plethora of functions mainly aimed at preserving the central nervous system (CNS) homeostasis. Glial cells are usually classified into microglia and macroglia. This latter includes astrocytes, oligodendrocytes, and oligodendrocyte precursors, also known as NG-2 glia or synantocytes [3, 4]. Microglia are of myeloid origin; their precursors migrate into the neural tube early in development. Microglial cells undergo profound metamorphosis acquiring specific 'neural'-like morphology and expressing numerous receptors for neurotransmitters and neurohormones [5]. Microglial cells contribute to CNS physiology and are mounting CNS defence in pathology being the main immunocompetent cells of the nervous tissue. Microglia scan the tissue and modify their morphology and functions if and when necessary [6]. Microglia are crucial for the formation, shaping, and functioning of synapses [7, 8], fundamental for brain development during pre- and post-natal periods. Erratic execution of synaptic elimination by microglia during early post-natal life is associated with anomalous functional connectivity, hippocampal long-term potentiation impairment, and aberrant behaviours [9, 10]. Microglia activate phagocytosis to incorporate waste products, cellular debris, and pathogens. They could react to pro-inflammatory stimuli by releasing cytokines, chemokines, and reactive oxygen and nitrogen species [11].
Oligodendrocytes are macroglial cells chiefly responsible for the formation of the myelin sheath around axons, thus being a fundamental element of the brain connectome [12]. Oligodendrocytes also support axons through cytoplasmic-rich myelinic channels that allow bidirectional movement of macromolecules under the myelin sheath [12,13,14]. Oligodendrocytes originate from precursor cells mainly localized in the ventricular zones of the brain, from which they migrate to colonise the developing CNS and became mature cells. This process starts shortly before birth and continues lifelong. The maturation of oligodendrocytes is usually accelerated in case of CSN injury, aging, or brain diseases, in order to replace the lost myelin [15]. Functions of oligodendrocyte precursors, also known as NG-2 glia, remain to be fully characterised. These cells express several receptors and ion channels, their receive synaptic contacts and may contribute to homeostatic control of the nervous tissue. Since NG-2 glia can differentiate into oligodendrocytes, they play a role in myelination and brain plasticity [16, 17].
Astroglia are fundamental for the maintenance of CNS homeostasis at molecular, cellular, organ, and system levels of organization [18, 19]. Several morphologically and functionally distinct subtypes of astroglial cells have been identified (e.g., protoplasmic and fibrous astrocytes of the grey matter, velate astrocytes of the cerebellum and olfactory bulb, radial astrocytes, perivascular and marginal astrocytes, ependymocytes, and many others) [19]. Astrocytes form the parenchymal part of the blood–brain barrier (BBB), which controls the exchanges of molecules and fluids between the brain and the periphery as well as restricts pathogens and cells brain invasion [18, 20,21,22]. Astrocytes regulate interstitial pH, control the concentration of extracellular ions, and scavenge reactive oxygen species [19]. Astrocytes are the central part of neuro-vascular unit, and they are involved in the regulation of local hyperaemia through the release of vasoactive molecules [23, 24]. Astrocytes are a part of the gliocrine system secreting about 200 molecules, including neurotrophic and synaptogenic factors as well as providing energy support to other neural cells [25]. Finally, astrocytes are fundamental components of the glymphatic system responsible for cleansing the nervous tissue [26]. Through morphological contact with synapses, astrocytes form the 'synaptic cradle', regulating all aspects of synaptic functions from synaptogenesis to synaptic maintenance and extinction [27]. In particular, astrocytes are indispensable for the control of neurotransmitter homeostasis in the brain [18, 28]. Astrocytes control so many cerebral functions that they are considered the homeostatic cells par excellence. As a consequence, any changes in the physiological performance of astrocytes may have a role in the etiology or progression of neurological pathologies.
Any insult to the CNS, including invasion of pathogens, triggers glial response, known as reactive astrogliosis [29]. Reactive glia play a fundamental defensive role, starting a series of responses aimed at restoring the lost homeostasis. Peripheral proinflammatory cytokines may induce microgliosis and astrogliosis whenever a CNS insult occurs, including viral infections [30]. Whenever glial cells lose their homeostatic activities, neuronal cells suffer. If the alteration of glial cells persists the irreversible damage to the nervous tissue may occur [3, 31,32,33,34].
The Neurotropism of SARS-CoV-2 and the Neurological Manifestations of COVID-19
SARS-CoV-2 which causes COVID-19 emerged from China in 2019. The virus spread rapidly through the world triggering the pandemic. SARS-CoV-2 is a positive-sense single-stranded β-coronavirus belonging to the family of Coronaviridae, the largest group of viruses causing respiratory and gastrointestinal infections [35, 36]. Historically, coronaviruses received little attention due to their scant effects on humans. This changed in 2002 when atypical pneumonia spread from the Guangdong province to more than twenty countries. This illness was named severe acute respiratory syndrome (SARS) and the identified etiological β-coronavirus was named SARS-CoV [37]. Although COVID-19 seems to have a lower case-fatality rate than SARS (about 2.3% versus about 6.4%, respectively [38]), the massive spread of the infection has claimed over 6.3 million casualties worldwide (WHO web-dashboard data updated on June 29th, 2022). The majority of the infected people appear to have eliminated the coronavirus from their bodies after a few weeks and resume normal activity. However, about the 40% of infected people experience a variety of symptoms (loss of smell and/or taste, fatigue, cough, aching pain, "brain fog," insomnia, shortness of breath, and tachycardia) after several weeks and are diagnosed with the so-called long COVID syndrome.
To invade cells the SARS-CoV-2 spike protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor which undergoes proteolytic processing by the transmembrane protease serine 2 [39,40,41]. In addition, both basigin (also known as CD147) and neuropilin-1 were identified as docking receptors for the SARS-CoV-2 virus [42,43,44]. After the first signs of the illness, the patient experiences a short recovery time, in which symptoms attenuate, usually followed by a more severe symptomatic. The human immune response induced by SARS-CoV-2 should develop in two phases. The constitutive adaptive immune response is activated at the beginning of the disease fighting the virus that actively replicates to colonise and damage the cells of the affected tissues, mostly lungs [38, 45]. A second phase, that take place in severe cases of COVID-19, defined as a severe acute respiratory distress syndrome (ARDS), is characterised by the so-called “cytokine storm” that is due to the hyperactivation of the immune system, accompanied by a massive release of proinflammatory mediators, cytokines, and chemokines [46, 47]. The hyperactive immune response impacts upon many organs and systems, underlying the multi-organ pathology observed in COVID-19 patients [48]. Thus, the cytokine load has also become the major hallmark in COVID-19 patients [49]. Growing clinical data suggest that patients having pre-existing conditions, such as obesity, cardiovascular diseases, hypertension, dyslipidemia, have a higher risk of developing severe or fatal COVID-19 [50,51,52].
Extrapulmonary manifestations of COVID-19 including neurological symptomatology, primarily anosmia and ageusia, are frequently reported [53,54,55]. Neurological symptoms in COVID-19 patients are grossly underestimated, especially because many severely ill patients are sedated and on ventilators [56]. However, cases of encephalitis, strokes, confusion, seizures, and brain inflammation have been reported [57,58,59]. A retrospective clinical study has provided evidence for substantial incidence of neurological and psychiatric events in patients during the first 6 months after getting COVID-19. The risk for neurological and psychiatric sequelae seems to be greatest in patients who had severe COVID-19 [60]. Cognitive deficits and depression have been seen in patients that recovered from mild COVID-19 [61].
The capability of SARS-CoV-2 to enter the CNS has been suggested by analogy with the neurotropism of other members of group 2 of the β-coronavirus family [62,63,64,65,66,67], to which SARS-CoV-2 belongs. Among several suggested routes of entry, the most studied and acknowledged is binding to ACE2 which is expressed in the CNS, mostly by endothelial cells [68] but also by both neurones and glial cells [69,70,71]. SARS-CoV-2 engages ACE2 as the entry receptor and employs the cellular serine protease TMPRSS2 for spike protein S cleavage [41]. This activates virus endocytosis controlled by endosomal proton pump and NAADP-sensitive intracellular two-pore channel 2 [72]. The ACE2 is expressed in the brain stem [69, 70], populating highly vascularised brain structures lacking the BBB like the circumventricular organs, the nucleus of the tractus solitarius, paraventricular nucleus, and rostral ventrolateral medulla [73]. Such distribution makes these regions more vulnerable to peripheral neurotoxic molecules or invasive agents, like SARS-CoV-2.
Another proposed route for viral entry to the brain is the invasion and consequent lesion of the olfactory system, which is consistent with the clinical data reporting that infection with SARS-CoV-2 is associated with high rates of disturbances in smell and taste perception, including anosmia [74,75,76,77,78]. Recently the cell types in the olfactory epithelium and olfactory bulb that express the SARS-CoV-2 cell entry molecules have been identified. Single-cell sequencing revealed that ACE2 is expressed in support cells, stem cells, and perivascular cells, rather than in neurons [79]. Through the olfactory system, the virus could spread into the brain stem, possibly compromising the respiratory centres [80]. Magnetic resonance imaging (MRI) investigations seem to corroborate that virus may enter the brain through the trans-nasal route [81,82,83,84], however, further studies are needed to better define their neuroradiologic interpretation.
Alternatively, the SARS-CoV-2 could penetrate through the median eminence, whose capillaries and tanycytes are thought to express ACE2, reaching the hypothalamus [85], and from there it could spread to the entire brain.
Brain infiltration of immune cells carrying the virus (a viral reservoir [86]) may represent another route of the virus entry. Vessels, meninges, and the choroid plexus have been proposed as entry points for infected monocytes, neutrophils, and T cells. However, conclusive evidence of infection through these routes has yet to be provided [87]. As suggested by some authors, some neurological symptoms and damage are the result of the body’s own immune system overreacting after encountering the virus. Some subjects inadvertently make ‘autoantibodies’ that attack their own tissue [88]. These autoantibodies can pass via the BBB, and contribute to both short- and long-term conditions, including neurological disorders ranging from brain fog to psychosis.
Evidence has also accumulated that the virus SARS-CoV-2 can affect the brain by reducing blood flow to it. In this way, SARS-CoV-2 infection impairs neurons function and ultimately killing them. Lastly, a leaky or dysfunctional BBB could facilitate the entry of the virus, as in other kinds of infections. For instance, the human immunodeficiency virus (HIV)-1 downregulates the expression of tight junction proteins, compromising BBB integrity [89]. Numerous studies highlight that systemic inflammation could damage glia limitans and damage the BBB [90]. Thus, the hyperreactive immune response triggered by SARS-CoV-2 may compromise the integrity of the BBB. Severe COVID-19 is often associated with comorbidities, such as CNS hypoxia due to respiratory failure, thrombotic microangiopathy, or pre-existing neurological diseases, which all may increase BBB permeability facilitating the entry of the virus into the brain [91]. The reported presence of SARS-CoV-2 in patients cerebrospinal fluid (CSF) and brain tissue suggests that once in the body the virus can reach the brain [92,93,94]. This is also true for SARS-CoV-1 [65, 66].Finally, the observation in post-mortem brain tissues of SARS-CoV-2 signal not coinciding with immune cell infiltration suggests that virus-related neurological complications could be the direct consequence of the neurotropism of SARS-CoV-2 [95, 96].
The astrocyte response to viruses, including SARS-CoV-2
Typically, whenever any virus enters the CNS, the innate immune response activates. Both immune and neural cells participate in this process, cooperating in removing the pathogen. Astrocytes control the communication between resident and infiltrating immune cells and regulate the effector functions of antiviral T and B cells in the CNS compartments [97, 98]. Astrocytes respond quickly to brain insults, viruses including, by virtue of their functions of monitoring and preserving the brain homeostasis. In response to brain insults, astrocytes initiate the programme of reactive astrogliosis generally characterised by increased levels of the intermediate filament proteins glial fibrillary acidic protein (GFAP), vimentin, and nestin, as well as by hypertrophy of astrocytic processes, although in some cases atrophy has been documented too. In specific conditions, such as acute trauma, astrocytes may proliferate, regulate scar formation by fibroblasts and form new barriers around lesioned foci [29, 31, 99]. Reactive astrocytes are generally neuroprotective because they amplify homoeostatic cascades, detect and remove toxic substances and promote regeneration. At the same time, during viral infections, astrocytes and microglia may also become long-term virus reservoirs in the absence of efficient innate immune-mediated clearing mechanisms [100]. Viruses-induced rise in interleukin(IL)-1β and tumor necrosis factor(TNF)-α may cause changes in the metabolic phenotype of astrocytes, resulting in reduced glycogen storage and lactate transport, fundamental for energy support for neurons [97, 101]. In HIV-1 infection, astroglia release cytokines and chemokines able to reduce viral replication. Concurrently, when HIV infects astrocytes, it impairs their functions by forcing them to produce viral proteins, thus causing neuronal damage [102,103,104,105]. Furthermore, HIV-1 infected astrocytes release membrane HIV-1 Tat protein triggering mitochondrial dysfunction and neuronal death [106]. Proinflammatory cytokines secreted by microglial cells may promote astrogliosis whenever a CNS insult occurs, including viral infections [30, 107]. Astrogliosis and microgliosis could lead both of the cell types to gain aberrant functions or lose fundamental ones, resulting in neuronal damage [29, 108].
Murine coronavirus, MHV-A59, could infect the brain and its CNS effects were mediated by the cytokine release from reactive microglia and astrocytes. The authors documented that the cytokines released from both cell types were complementary, resulting in elevated levels of IL-1β, IL-6, interferon(INF)s, and TNF-α. Of note, they did not detect the release of the anti-inflammatory cytokines IL-4 and IL-10 [109]. In a SARS-CoV-1 patient, necrosis of neurons, broad hyperplasia of glial cells, and encephalic oedema have been reported. High plasma level of the chemokine Mig, a monokine induced by the INF-γ, that promotes the host defence by attracting activated T cells, natural killer (NK) cells, and CXCR3 expressing monocytes [66] has also been detected. Several studies indicated that SARS-CoV-2 affects astrocytes. A recent post-mortem investigation demonstrated that astrocytes are the main sites of viral infection within the CNS and that SARS-CoV-2-infected cells exhibit marked metabolic changes [110]. These authors suggest that astrocyte functions are impaired since they detected a reduction of the metabolites used to fuel neurons and produce neurotransmitters. In cortical tissue cultures and cortical organoids exposed to SARS-CoV-2, it has recently been demonstrated significant infection and viral replication in astrocytes, but minimal infection in other cell types [111].The same group reported that infected astrocytes had a corresponding increase in reactivity characteristics, growth factor signaling, and cellular stress. Signs of astrocyte reactivity have long been proposed in COVID-19 patients. For instance, elevated GFAP was found in the white matter of a COVID-19 patient, with encephalomyelitis-like brain damage, oligodendrocytic apoptosis and axonal injuries [112]. Plasma levels of both GFAP and neurofilament light chain protein (NfL), a biomarker predictive of intra-axonal neuronal injury, were measured in 47 patients with mild, moderate, or severe COVID-19 and matched controls. GFAP was found elevated in moderate/severe stages of the disease. This suggests that astrogliosis could be an early response after SARS-CoV-2 infection of the CNS [113]. In COVID-19-related acute necrotising encephalopathy virus was detected in the CSF, together with extremely high levels of NfL and GFAP, 19 days after the onset of the symptoms and even after testing negative twice [94]. These clinical studies indicate that astrocytes could be in a reactive state in COVID-19 patients. Consistently, the damage of the BBB and the strong lymphopenia observed during COVID-19 could promote the persistence of the virus, thus sustaining neuroinflammation and reactive gliosis. The resulting brain tissue alteration could explain some of the clinical features observed in COVID-19 patients who, despite overcome pneumonia, present cognitive impairment associated with behavioural changes [2, 114,115,116,117].
Neuropsychiatric Consequences of COVID-19
CNS viral infections induce cognitive, mood, and motor deficits that may persist beyond the acute phase of the disease. In many cases, CNS sequelae may be provoked by irreversible damage to both neurons and glia triggered by pathogens [118]. Otherwise, infection-driven neuroinflammation can disturb brain homeostasis and circuit functioning inducing long-term deficits resulting in behavior alterations [119]. In this context, focusing on the neuropsychiatric sequelae that emerged following the SARS -COV 2 infection, longitudinal epidemiology research has revealed a broad spectrum of long-term consequences in patients who survived to COVID-19 pandemic, providing evidence that almost 80% of subjects discharged from hospital complained at least one of the following symptoms including fatigue, muscle weakness, myalgia, dizziness, headache sleep disturbances, brain fog, cognitive impairment, depression or anxiety in addition to cardiopulmonary manifestations [120,121,122]. Inevitably, the frequent persistence of this condition up to six months and beyond together with the failure of any effective treatment has a considerable impact on the quality of life of the affected subjects, keeping them out of work and social life [123]. Evidence that COVID-19 is followed by a significant rate of neuropsychiatric diagnoses over the subsequent six months has been further confirmed by a robust retrospective cohort study [117]. On the basis of this data, particular interest was drawn from the persistence of neuropsychiatric symptoms in convalescent patients or from their late appearance in subjects completely restored by the viral infection [60, 124, 125]. This should not be surprising, as similar features with significant neurological and mental complains were already reported in acute or post-disease phases during other previous coronavirus outbreaks. The experience gained with neurological and psychiatric manifestations of MERS and SARS would provide the right framework for better exploring CNS complications occurring during SARS-CoV-2 infection [126]. Post-COVID-19 psychiatric pathology frequently begins with a fatiguing feeling of asthenia, with a sense of apathy resulting in a condition of reduced interest in interpersonal relationships, and a decreased pleasure in carrying out those occupations that were previously a source of satisfaction. The appearance of sleep–wake rhythm disturbances and a progressive decrease in mood testify to the onset of an overt depression [127]. Depression following pandemics is considered one of the most significant public health concerns. A recent study reported a long-term prevalence beyond twelve months of 18.3% [120], while another investigation on the same topic suggested an overall depression prevalence of 27.9% [128]. According to some authors, the depression observed in the condition defined as long COVID appears to be characterized by manifestations that distinguish it from the canonical major depressive disorder [119]. Highlights of post-COVID-19 depression include a higher incidence of psychotic traits, marked motor agitation, evident neurocognitive deterioration, and profound changes in the sleep–wake rhythm. Psychotic anomalies, consisting in delusions, hallucinations, thoughts, disorganized may initially appear at the height of the COVID-19 pathology, and may also persist when the delirium is over and the infection is resolved. Psychotic manifestations many times emerge weeks or months after healing from the infection, not accompanied by delirium or confusion, mimicking the onset of a primary psychotic episode. Sleep disorders frequently complicate the clinical picture of long COVID-19 and are characterized by marked difficulty in initiating sleep rather than keeping it uninterrupted [129]. These sleep disturbances frequently occur in the younger population experiencing COVID-19 if even asymptomatic, without significant anxiety levels about the outcome and consequences of the infection. Sometimes insomnia remains even beyond the disappearance of the other disorders that had characterized the long COVID-19, in the absence of a manifest anxiety and an overt decline in mood, therefore leaving insomnia without a clear explanation [2, 129, 130]. A recent report by Jahrami et al. assessing the impact of the COVID-19 pandemic on the prevalence and severity of sleep problems among patients with COVID-19 indicated a high frequency of disturbed sleep, with an average rate of 74.8% [131]. Such an important incidence of sleep disturbances in patients who recovered from the acute SARS-CoV-2 infection could be explained by the interaction between sleep impairment and immune system dysfunction [132]. In fact, sleep and the immune system, according to researchers, interact bidirectionally. This hypothesis is supported by altered sleep patterns during viral infections with the release of inflammatory molecules, particularly in the acute phase of the immune response and the development of recovery during sickness [133]. The response of the immune system to infection, with the subsequent release of these immunological mediators, results in the activation of glial cells which consequently lose their modulatory role in the sleep homeostasis [134]. Moreover, at the level of mental disorders, there is a priori reason to expect that at least a substantial proportion of patients with obsessive–compulsive disorder (OCD) would experience a worsening of their disturbances due to the pandemic, with contamination/washing symptoms being the most susceptible [135]. Indeed, stressful life events may precipitate or predispose individuals to the development of OCD symptoms. The intense focus on the potential danger of contamination, as well as the COVID 19 infection, may induce the onset of OCD manifestations in vulnerable subjects, even after months of healing from the disease [136]. Currently, except for epidemiological findings, there are no studies specifically aimed at establishing whether and how COVID-19 infection itself could lead to de novo OCD symptoms or exacerbation of symptoms in people with OCD. In this regard, it is important to take into account the stressful effects of the pandemic, but also it is crucial to consider that infective and/or inflammatory processes have been implicated in some cases of OCD-like symptoms [137], with the evidence of a glial activation occurring in the neurocircuits of OCD [138]. Similarly, de novo appearance of post-traumatic stress disorder (PTSD) spectrum symptoms or their worsening in people who experienced COVID 19 infection is not surprising since the links between inflammation, immune system alterations, and stress-related diseases have been widely demonstrated [139].
Therefore, the risk of increased prevalence of PTSD has also been observed in previous coronavirus pandemics, making its occurrence during this COVID-19 pandemic highly explainable. Some severe cases of COVID-19 result in mortality. The fear of death might be among the many reasons responsible for PTSD amongst these patients. It has been demonstrated that 16% of the severe COVID-19 patients globally had PTSD [140].
A meta-analysis of the survivors among emergency-admitted patients with SARS and MERS infection has revealed that about 39% of them had suffered from PTSD. A history of psychiatric disorders, especially anxiety and depressive disorders, was found as a risk factor for PTSD in intensive care unit survivors. Twelve months after infection, psychiatric symptoms among COVID-19 recovered survivors were reported as 18.3% for depression, 17.9% for PTSD, 16.2% for anxiety, and 13.5% for sleep disturbance [120].
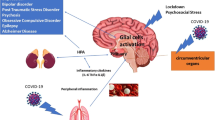
Based on the above reported findings it is possible to state that psychiatric involvement is not uncommon and can lead to severe problems if not detected and managed early. It is recommended that clinicians should be vigilant regarding psychiatric involvement in post COVID-19 patients. Neuroinflammation, blood–brain barrier disruption, thrombotic events, peripheral immune cell invasion into the CNS, glial activation, brain homeostasis impairment, all represent interaction pathways between immune systems and psychopathological mechanisms underpinning such disorders (Fig. 1). In support of this, a recent analysis of brain images taken before and after infection with SARS-CoV-2 demonstrated that even mild COVID-19 is associated with brain structure alterations and brain functioning impairments, suggesting that the effects of SARS-CoV-2 on the CNS need to be very seriously considered [141].
Although evidence suggests that the virus can enter the brain, it seems, however, to predominantly infect vascular and immune cells. In response to immune over activation (and/or virus direct invasion) astrocytes and microglia become reactive. Also, hemorrhages, microvascular infarcts, and thrombotic events are probably critical in the development of the neurological manifestations of SARS-CoV-2 infection.
Conclusions
COVID-19, initially regarded as specific lung disease, impacts many other organs, affecting their function. Several underlying medical conditions that increase the risk of severe COVID-19 have been identified [142]. Evidence accumulated in the past two years indicates that the brain functions and structure are also damaged by the virus. SARS-CoV-2 infection can indeed cause confusion, memory loss, strokes, psychosis, seizures, and other neurological manifestations. There is also evidence of brain-related abnormalities in COVID-19 patients [141] that may explain the neurological manifestations observed. A study revealed that neurological symptoms appeared in 80% of the people hospitalized with COVID-19 who were surveyed [143]. This depicts a dramatic scenario.
How COVID-19 injuries the brain is becoming clearer. New evidence indicates that the SARS-CoV-2 assault on the brain could be multipronged. The coronavirus might target specific brain cells directly, reduce cerebral blood flow, or trigger the production of immune molecules that can harm brain cells. Astrocytes, tanycytes, infiltrating immune cells, and autoantibodies are probably not the only players in the brain response to the coronavirus leading to the observed neuropsychiatric consequences. Researchers are trying to understand how many brain cells (and what kind of cells) need to be either infected or damaged to cause neurological symptoms. Unfortunately, there isn’t a simple answer. Cerebral cells, including neurons, in some regions of the brain will cause more dysfunction than others, if damaged. This opens a new and never explored field of research. Lastly, whether cerebral effects can be partially reversed, or whether these effects will persist in the long term, remains to be investigated.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Valenza M, Steardo L Jr, Steardo L et al (2021) Systemic inflammation and astrocyte reactivity in the neuropsychiatric sequelae of COVID-19: focus on autism spectrum disorders. Front Cell Neurosci 15:748136. https://doi.org/10.3389/fncel.2021.748136
Steardo L Jr, Steardo L, Verkhratsky A et al (2021) Post-COVID-19 neuropsychiatric syndrome: is maladaptive glial recovery to blame? Acta Physiol (Oxf). https://doi.org/10.1111/apha.13717
Butt A, Verkhratsky A (2018) Neuroglia: realising their true potential. Brain Neurosci Adv. https://doi.org/10.1177/2398212818817495
Verkhratsky A, Butt A (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester
Garaschuk O, Verkhratsky A (2019) Microglia: the neural cells of nonneural origin. Methods Mol Biol 2034:3–11. https://doi.org/10.1007/978-1-4939-9658-2_1
Kettenmann H, Hanisch UK, Noda M et al (2011) Physiology of microglia. Physiol Rev 91:461–553. https://doi.org/10.1152/physrev.00011.2010
Paolicelli RC, Bolasco G, Pagani F et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. https://doi.org/10.1126/science.1202529
Schafer DP, Lehrman EK, Kautzman AG et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. https://doi.org/10.1016/j.neuron.2012.03.026
Zhan Y, Paolicelli RC, Sforazzini F et al (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406. https://doi.org/10.1038/nn.3641
Zeidan-Chulia F, Salmina AB, Malinovskaya NA et al (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172. https://doi.org/10.1016/j.neubiorev.2013.11.008
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Butt AM, Papanikolaou M, Rivera A (2019) Physiology of oligodendroglia. Adv Exp Med Biol 1175:117–128. https://doi.org/10.1007/978-981-13-9913-8_5
Lasiene J, Matsui A, Sawa Y et al (2009) Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8:201–213. https://doi.org/10.1111/j.1474-9726.2009.00462.x
Simons M, Nave KA (2015) Oligodendrocytes: myelination and axonal support. Cold Spring Harb Perspect Biol 8:a020479. https://doi.org/10.1101/cshperspect.a020479
Bergles DE, Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol 8:a020453. https://doi.org/10.1101/cshperspect.a020453
Eugenin-von Bernhardi J, Dimou L (2016) NG2-glia, more than progenitor cells. Adv Exp Med Biol 949:27–45. https://doi.org/10.1007/978-3-319-40764-7_2
Du X, Zhang Z, Zhou H et al (2021) Differential modulators of NG2-Glia differentiation into neurons and glia and their crosstalk. Cell Mol Neurobiol 41:1–15. https://doi.org/10.1007/s10571-020-00843-0
Verkhratsky A, Nedergaard M, Hertz L (2015) Why are astrocytes important? Neurochem Res 40:389–401. https://doi.org/10.1007/s11064-014-1403-2
Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389. https://doi.org/10.1152/physrev.00042.2016
Valenza M, Facchinetti R, Steardo L et al (2019) Altered waste disposal system in aging and Alzheimer’s disease: focus on astrocytic aquaporin-4. Front Pharmacol 10:1656. https://doi.org/10.3389/fphar.2019.01656
Pivoriunas A, Verkhratsky A (2021) Astrocyte-endotheliocyte axis in the regulation of the blood-brain barrier. Neurochem Res. https://doi.org/10.1007/s11064-021-03338-6
Sweeney MD, Zhao Z, Montagne A et al (2019) Blood-brain barrier: from physiology to disease and back. Physiol Rev 99:21–78. https://doi.org/10.1152/physrev.00050.2017
Parpura V, Verkhratsky A (2012) Neuroglia at the crossroads of homoeostasis, metabolism and signalling: evolution of the concept. ASN Neuro 4:201–205. https://doi.org/10.1042/AN20120019
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96:17–42. https://doi.org/10.1016/j.neuron.2017.07.030
Verkhratsky A, Matteoli M, Parpura V et al (2016) Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35:239–257
Iliff JJ, Wang M, Liao Y et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. https://doi.org/10.1126/scitranslmed.3003748
Verkhratsky A, Nedergaard M (2014) Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci 369:20130595. https://doi.org/10.1098/rstb.2013.0595
Fields RD, Woo DH, Basser PJ (2015) Glial regulation of the neuronal connectome through local and long-distant communication. Neuron 86:374–386. https://doi.org/10.1016/j.neuron.2015.01.014
Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325. https://doi.org/10.1038/s41593-020-00783-4
Zorec R, Zupanc TA, Verkhratsky A (2019) Astrogliopathology in the infectious insults of the brain. Neurosci Lett 689:56–62. https://doi.org/10.1016/j.neulet.2018.08.003
Scuderi C, Stecca C, Iacomino A et al (2013) Role of astrocytes in major neurological disorders: the evidence and implications. IUBMB Life 65:957–961. https://doi.org/10.1002/iub.1223
Pekny M, Pekna M (2016) Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862:483–491. https://doi.org/10.1016/j.bbadis.2015.11.014
Valenza M, Facchinetti R, Menegoni G et al (2021) Alternative targets to fight Alzheimer’s disease: focus on astrocytes. Biomolecules. https://doi.org/10.3390/biom11040600
Verkhratsky A, Zorec R, Parpura V (2017) Stratification of astrocytes in healthy and diseased brain. Brain Pathol 27:629–644. https://doi.org/10.1111/bpa.12537
de Wit E, van Doremalen N, Falzarano D et al (2016) SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14:523–534. https://doi.org/10.1038/nrmicro.2016.81
Paules CI, Marston HD, Fauci AS (2020) Coronavirus infections-more than just the common cold. JAMA 323:707–708. https://doi.org/10.1001/jama.2020.0757
Zhong NS, Zheng BJ, Li YM et al (2003) Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 362:1353–1358. https://doi.org/10.1016/s0140-6736(03)14630-2
Gerges Harb J, Noureldine HA, Chedid G et al (2020) SARS, MERS and COVID-19: clinical manifestations and organ-system complications: a mini review. Pathog Dis. https://doi.org/10.1093/femspd/ftaa033
Zhang H, Penninger JM, Li Y et al (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590. https://doi.org/10.1007/s00134-020-05985-9
Zhou P, Yang XL, Wang XG et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(271–280):e278. https://doi.org/10.1016/j.cell.2020.02.052
Wang K, Chen W, Zhang Z et al (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5:283. https://doi.org/10.1038/s41392-020-00426-x
Cantuti-Castelvetri L, Ojha R, Pedro LD et al (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370:856–860. https://doi.org/10.1126/science.abd2985
Murgolo N, Therien AG, Howell B et al (2021) SARS-CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog 17:e1009225. https://doi.org/10.1371/journal.ppat.1009225
Shi Y, Wang Y, Shao C et al (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27:1451–1454. https://doi.org/10.1038/s41418-020-0530-3
Polidoro RB, Hagan RS, de Santis SR et al (2020) Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol 11:1626. https://doi.org/10.3389/fimmu.2020.01626
Jamilloux Y, Henry T, Belot A et al (2020) Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 19:102567. https://doi.org/10.1016/j.autrev.2020.102567
Moore JB, June CH (2020) Cytokine release syndrome in severe COVID-19. Science 368:473–474. https://doi.org/10.1126/science.abb8925
Abdin SM, Elgendy SM, Alyammahi SK et al (2020) Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci 257:118054. https://doi.org/10.1016/j.lfs.2020.118054
Badawi A, Ryoo SG (2016) Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis 49:129–133. https://doi.org/10.1016/j.ijid.2016.06.015
Chen X, Zhao B, Qu Y et al (2020) Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically Ill patients with coronavirus disease 2019. Clin Infect Dis 71:1937–1942. https://doi.org/10.1093/cid/ciaa449
Wu C, Chen X, Cai Y et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:934–943. https://doi.org/10.1001/jamainternmed.2020.0994
Deng J, Zhou F, Hou W et al (2021) The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci 1486:90–111. https://doi.org/10.1111/nyas.14506
Fernandez-de-Las-Penas DC (2021) Anxiety, depression and poor sleep quality as long-term post-COVID sequelae in previously hospitalized patients: a multicenter study. J Infect. https://doi.org/10.1016/j.jinf.2021.06.022
Vaira LA, Salzano G, Deiana G et al (2020) Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 130:1787. https://doi.org/10.1002/lary.28692
Wadman M, Couzin-Frankel J, Kaiser J et al (2020) A rampage through the body. Science 368:356–360. https://doi.org/10.1126/science.368.6489.356
Asadi-Pooya AA, Simani L (2020) Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 413:116832. https://doi.org/10.1016/j.jns.2020.116832
Desforges M, Le Coupanec A, Dubeau P et al (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. https://doi.org/10.3390/v12010014
Hugon J, Msika EF, Queneau M et al (2021) Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. https://doi.org/10.1007/s00415-021-10655-x
Taquet M, Geddes JR, Husain M et al (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8:416–427. https://doi.org/10.1016/s2215-0366(21)00084-5
Woo MS, Malsy J, Pottgen J et al (2020) Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun 2:f205. https://doi.org/10.1093/braincomms/fcaa205
Tavcar P, Potokar M, Kolenc M et al (2021) Neurotropic viruses, astrocytes, and COVID-19. Front Cell Neurosci 15:662578. https://doi.org/10.3389/fncel.2021.662578
Bohmwald K, Galvez NMS, Rios M et al (2018) Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 12:386. https://doi.org/10.3389/fncel.2018.00386
Bergmann CC, Lane TE, Stohlman SA (2006) Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol 4:121–132. https://doi.org/10.1038/nrmicro1343
Lau KK, Yu WC, Chu CM et al (2004) Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 10:342–344. https://doi.org/10.3201/eid1002.030638
Xu J, Zhong S, Liu J et al (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41:1089–1096. https://doi.org/10.1086/444461
Zhou Z, Kang H, Li S et al (2020) Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol 267:2179–2184. https://doi.org/10.1007/s00415-020-09929-7
Zeisel A, Munoz-Manchado AB, Codeluppi S et al (2015) Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347:1138–1142. https://doi.org/10.1126/science.aaa1934
Xia H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12:170–175. https://doi.org/10.1007/s11906-010-0105-7
Gowrisankar YV, Clark MA (2016) Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem 138:74–85. https://doi.org/10.1111/jnc.13641
Nemoto W, Yamagata R, Nakagawasai O et al (2020) Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur J Pharmacol 872:172950. https://doi.org/10.1016/j.ejphar.2020.172950
Petersen OH, Gerasimenko OV, Gerasimenko JV (2020) Endocytic uptake of SARS-CoV-2: the critical roles of pH, Ca2+, and NAADP. Function. https://doi.org/10.1093/function/zqaa003
Duvernoy HM, Risold PY (2007) The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev 56:119–147. https://doi.org/10.1016/j.brainresrev.2007.06.002
Giacomelli A, Pezzati L, Conti F et al (2020) Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 71:889–890. https://doi.org/10.1093/cid/ciaa330
Wolfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. https://doi.org/10.1038/s41586-020-2196-x
Lechien JR, Chiesa-Estomba CM, De Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277:2251–2261. https://doi.org/10.1007/s00405-020-05965-1
Menni C, Valdes AM, Freidin MB et al (2020) Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 26:1037–1040. https://doi.org/10.1038/s41591-020-0916-2
Spinato G, Fabbris C, Polesel J et al (2020) Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 323:2089–2090. https://doi.org/10.1001/jama.2020.6771
Brann DH, Tsukahara T, Weinreb C et al (2020) Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. https://doi.org/10.1126/sciadv.abc5801
Li K, Wohlford-Lenane C, Perlman S et al (2016) Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 213:712–722. https://doi.org/10.1093/infdis/jiv499
Coolen T, Lolli V, Sadeghi N et al (2020) Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 95:e2016–e2027. https://doi.org/10.1212/WNL.0000000000010116
Laurendon T, Radulesco T, Mugnier J et al (2020) Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 95:224–225. https://doi.org/10.1212/WNL.0000000000009850
Politi LS, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 77:1028–1029. https://doi.org/10.1001/jamaneurol.2020.2125
Li CW, Syue LS, Tsai YS et al (2021) Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect 54:93–96. https://doi.org/10.1016/j.jmii.2020.05.017
Satarker S, Nampoothiri M (2020) Involvement of the nervous system in COVID-19: The bell should toll in the brain. Life Sci 262:118568. https://doi.org/10.1016/j.lfs.2020.118568
Iadecola C, Anrather J, Kamel H (2020) Effects of COVID-19 on the nervous system. Cell 183(16–27):e11. https://doi.org/10.1016/j.cell.2020.08.028
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362. https://doi.org/10.1038/s41577-020-0331-4
Franke C, Ferse C, Kreye J et al (2021) High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 93:415–419. https://doi.org/10.1016/j.bbi.2020.12.022
Cisneros IE, Ghorpade A (2012) HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res 10:392–406. https://doi.org/10.2174/157016212802138832
Sankowski R, Mader S, Valdes-Ferrer SI (2015) Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 9:28. https://doi.org/10.3389/fncel.2015.00028
Erickson MA, Rhea EM, Knopp RC et al (2021) Interactions of SARS-CoV-2 with the blood-brain barrier. Int J Mol Sci. https://doi.org/10.3390/ijms22052681
Huang YH, Jiang D, Huang JT (2020) SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun 87:149. https://doi.org/10.1016/j.bbi.2020.05.012
Paniz-Mondolfi A, Bryce C, Grimes Z et al (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 92:699–702. https://doi.org/10.1002/jmv.25915
Virhammar J, Kumlien E, Fallmar D et al (2020) Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 95:445–449. https://doi.org/10.1212/WNL.0000000000010250
Song E, Zhang C, Israelow B et al (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. https://doi.org/10.1084/jem.20202135
Zhang Y, Archie SR, Ghanwatkar Y et al (2022) Potential role of astrocyte angiotensin converting enzyme 2 in the neural transmission of COVID-19 and a neuroinflammatory state induced by smoking and vaping. Fluids Barriers CNS 19:46. https://doi.org/10.1186/s12987-022-00339-7
Soung A, Klein RS (2018) Viral encephalitis and neurologic diseases: focus on astrocytes. Trends Mol Med 24:950–962. https://doi.org/10.1016/j.molmed.2018.09.001
Klein RS, Garber C, Funk KE et al (2019) Neuroinflammation during RNA viral infections. Annu Rev Immunol 37:73–95. https://doi.org/10.1146/annurev-immunol-042718-041417
Wahane S, Sofroniew MV (2021) Review article for CTR special issue edited by C. Schachtrup Title of Special Issue: "Modulating scar formation for improving brain repair" Loss-of-function manipulations to identify roles of diverse glia and stromal cells during CNS scar formation. Cell Tissue Res. https://doi.org/10.1007/s00441-021-03487-8
Muffat J, Li Y, Omer A et al (2018) Human induced pluripotent stem cell-derived glial cells and neural progenitors display divergent responses to Zika and dengue infections. Proc Natl Acad Sci U S A 115:7117–7122. https://doi.org/10.1073/pnas.1719266115
Gavillet M, Allaman I, Magistretti PJ (2008) Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia 56:975–989. https://doi.org/10.1002/glia.20671
Li W, Henderson LJ, Major EO et al (2011) IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol 186:6771–6778. https://doi.org/10.4049/jimmunol.1100099
Appay V, Rowland-Jones SL (2001) RANTES: a versatile and controversial chemokine. Trends Immunol 22:83–87. https://doi.org/10.1016/s1471-4906(00)01812-3
Zhou BY, Liu Y, Kim B et al (2004) Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci 27:296–305. https://doi.org/10.1016/j.mcn.2004.07.003
Williams R, Yao H, Dhillon NK et al (2009) HIV-1 Tat co-operates with IFN-gamma and TNF-alpha to increase CXCL10 in human astrocytes. PLoS ONE 4:e5709. https://doi.org/10.1371/journal.pone.0005709
Chauhan A, Turchan J, Pocernich C et al (2003) Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem 278:13512–13519. https://doi.org/10.1074/jbc.M209381200
Yadav A, Collman RG (2009) CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmun Pharmacol 4:430–447. https://doi.org/10.1007/s11481-009-9174-2
Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. https://doi.org/10.1101/cshperspect.a020420
Lavi E, Cong L (2020) Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp Mol Pathol 115:104474. https://doi.org/10.1016/j.yexmp.2020.104474
Crunfli F, Carregari VC, Veras FP et al (2022) Morphological, cellular and molecular basis of brain infection in COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.10.09.20207464
Andrews MG, Mukhtar T, Eze UC et al (2021) Tropism of SARS-CoV-2 for developing human cortical astrocytes. bioRxiv. https://doi.org/10.1101/2021.01.17.427024
Reichard RR, Kashani KB, Boire NA et al (2020) Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 140:1–6. https://doi.org/10.1007/s00401-020-02166-2
Kanberg N, Ashton NJ, Andersson LM et al (2020) Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 95:e1754–e1759. https://doi.org/10.1212/WNL.0000000000010111
Sasannejad C, Ely EW, Lahiri S (2019) Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care 23:352. https://doi.org/10.1186/s13054-019-2626-z
Boldrini M, Canoll PD, Klein RS (2021) How COVID-19 affects the brain. JAMA Psychiat 78:682–683. https://doi.org/10.1001/jamapsychiatry.2021.0500
Tremblay ME, Madore C, Bordeleau M et al (2020) Neuropathobiology of COVID-19: the role for glia. Front Cell Neurosci 14:592214. https://doi.org/10.3389/fncel.2020.592214
Steardo L Jr, Steardo L, Verkhratsky A (2020) Psychiatric face of COVID-19. Transl Psychiatry 10:261. https://doi.org/10.1038/s41398-020-00949-5
Steardo L, Steardo L Jr, Zorec R et al (2020) Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol (Oxf) 229:e13473. https://doi.org/10.1111/apha.13473
Steardo L Jr, Steardo L, Verkhratsky A et al (2021) Post-COVID-19 neuropsychiatric syndrome: Is maladaptive glial recovery to blame? Acta Physiol (Oxf) 233:e13717
Zeng N, Zhao YM, Yan W et al (2022) A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01614-7
Nehme M, Braillard O, Chappuis F et al (2021) Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med 174:1252–1260. https://doi.org/10.7326/m21-0878
Al-Aly Z, Bowe B, Xie Y (2022) Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. https://doi.org/10.1038/s41591-022-01840-0
Magnúsdóttir I, Lovik A, Unnarsdóttir AB et al (2022) Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health 7:e406–e416. https://doi.org/10.1016/s2468-2667(22)00042-1
Leung CMC, Ho MK, Bharwani AA et al (2022) Mental disorders following COVID-19 and other epidemics: a systematic review and meta-analysis. Transl Psychiatry 12:205. https://doi.org/10.1038/s41398-022-01946-6
Premraj L, Kannapadi NV, Briggs J et al (2022) Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci 434:120162. https://doi.org/10.1016/j.jns.2022.120162
Ahmed H, Patel K, Greenwood DC et al (2020) Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 52:jrm00063. https://doi.org/10.2340/16501977-2694
Ceban F, Ling S, Lui LMW et al (2022) Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun 101:93–135. https://doi.org/10.1016/j.bbi.2021.12.020
Shi L, Lu ZA, Que JY et al (2020) Prevalence of and risk factors associated with mental health symptoms among the general population in china during the coronavirus disease 2019 pandemic. JAMA Netw Open 3:e2014053. https://doi.org/10.1001/jamanetworkopen.2020.14053
Nowakowski S, Kokonda M, Sultana R et al (2022) Association between sleep quality and mental health among patients at a post-COVID-19 recovery clinic. Brain Sci. https://doi.org/10.3390/brainsci12050586
Pérez-Carbonell L, Meurling IJ, Wassermann D et al (2020) Impact of the novel coronavirus (COVID-19) pandemic on sleep. J Thorac Dis 12:s163–s175
Jahrami H, BaHammam AS, Bragazzi NL et al (2021) Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med 17:299–313. https://doi.org/10.5664/jcsm.8930
Dehghani A, Zokaei E, Kahani SM et al (2022) The potential impact of Covid-19 on CNS and psychiatric sequels. Asian J Psychiatr 72:103097. https://doi.org/10.1016/j.ajp.2022.103097
Kolokolov OV, Salina EA, Yudina VV et al (2022) Infections, pandemics, and sleep disorders. Neurosci Behav Physiol 52:319–325. https://doi.org/10.1007/s11055-022-01242-2
Broadhead MJ, Miles GB (2021) A common role for astrocytes in rhythmic behaviours? Prog Neurobiol 202:102052. https://doi.org/10.1016/j.pneurobio.2021.102052
Grant JE, Drummond L, Nicholson TR et al (2022) Obsessive-compulsive symptoms and the Covid-19 pandemic: a rapid scoping review. Neurosci Biobehav Rev 132:1086–1098. https://doi.org/10.1016/j.neubiorev.2021.10.039
Van Ameringen M, Patterson B, Turna J et al (2022) Obsessive-compulsive disorder during the COVID-19 pandemic. J Psychiatr Res 149:114–123. https://doi.org/10.1016/j.jpsychires.2022.02.001
Endres D, Pollak TA, Bechter K et al (2022) Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype? Transl Psychiatry 12:5. https://doi.org/10.1038/s41398-021-01700-4
Attwells S, Setiawan E, Wilson AA et al (2017) Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiat 74:833–840. https://doi.org/10.1001/jamapsychiatry.2017.1567
Lee DH, Lee JY, Hong DY et al (2022) Neuroinflammation in post-traumatic stress disorder. Biomedicines. https://doi.org/10.3390/biomedicines10050953
Yuan K, Gong YM, Liu L et al (2021) Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol Psychiatry 26:4982–4998. https://doi.org/10.1038/s41380-021-01036-x
Douaud G, Lee S, Alfaro-Almagro F et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. medRxiv. https://doi.org/10.1101/2021.06.11.21258690
Wei SQ, Bilodeau-Bertrand M, Liu S et al (2021) The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193:E540–E548. https://doi.org/10.1503/cmaj.202604
Chou SH, Beghi E, Helbok R et al (2021) Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open 4:e2112131. https://doi.org/10.1001/jamanetworkopen.2021.12131
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
LS, LS, and CS conceived the review manuscript and contributed to the writing and revision of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steardo, L., Steardo, L. & Scuderi, C. Astrocytes and the Psychiatric Sequelae of COVID-19: What We Learned from the Pandemic. Neurochem Res 48, 1015–1025 (2023). https://doi.org/10.1007/s11064-022-03709-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03709-7