Abstract
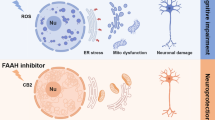
Zinc is highly enriched in the central nervous system. Numerous evidences suggest that high concentration of zinc acts as a critical mediator of neuronal death in the ischemic brain, however, the possible mechanisms of neurotoxicity of zinc during cerebral ischemia/reperfusion (I/R) remain elusive. Endoplasmic reticulum (ER) is a storage location of intracellular zinc. ER stress related genes were up-regulated during zinc-induced neuronal death in vascular-type senile dementia. In the present study, we investigated whether intracellular accumulated zinc aggravates I/R injury through ER stress and ER stress-associated apoptosis. Male Sprague–Dawley rats were subjected to 90 min middle cerebral artery occlusion (MCAO) and received either vehicle or zinc chelator TPEN 15 mg/kg. The expression of ER stress related factors glucose-regulated protein 78 (GRP78) and phosphorylated eukaryotic initiation factor 2α (p-eIF2α), ER stress related apoptotic proteins CCAAT-enhancer-binding protein homologous protein (CHOP) and caspase-12, as well as anti-apoptotic factor B-cell lymphoma-2 (Bcl-2) were assessed 24 h after reperfusion. Our results showed that the levels of GRP78 and p-eIF2α, as well as CHOP and caspase-12, were increased in ischemic brain, indicating that cerebral I/R triggers ER stress. Furthermore, GRP78, CHOP and caspase-12 were all colocalized with the zinc-specific dyes NG, suggesting that there is certain relationship between cytosolic labile zinc and ER stress following cerebral ischemia. Chelating zinc with TPEN reversed the expression of GRP78, p-eIF2α in ischemic rats. Moreover, CHOP and NeuN double staining positive cells, as well as caspase-12 and TUNEL double staining positive cells were also decreased after TPEN treatment, indicating that chelating zinc might inhibit ER stress and decreased ER stress associated neuronal apoptosis. In addition, TPEN treatment reversed the downregulated level of Bcl-2, which localized in the ER membrane and involved in the dysfunction of ER, confirming that the anti-apoptosis effects of chelating zinc following I/R are exerted via inhibition of the ER stress. Taken together, this study demonstrated that excessive zinc activates ER stress and zinc induced neuronal cell death is at least partially due to ER stress specific neuronal apoptosis in ischemic penumbra, which may provide an important mechanism of cerebral I/R injury.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1016
Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW (2002) Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 115:871–878
Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH (2003) Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A 100:6157–6162
Zhao Y, Yan F, Yin J, Pan R, Shi W, Qi Z, Fang Y, Huang Y, Li S, Luo Y, Ji X, Liu KJ (2018) Synergistic interaction between zinc and reactive oxygen species amplifies ischemic brain injury in rats. Stroke 49:2200–2210
Pan R, Chen C, Liu WL, Liu KJ (2013) Zinc promotes the death of hypoxic astrocytes by upregulating hypoxia-induced hypoxia-inducible factor-1alpha expression via poly(ADP-ribose) polymerase-1. CNS Neurosci Ther 19:511–520
Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, Qi Z, Yan Y, Ji X, Liu KJ (2014) Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke 45:1139–1147
Dong W, Qi Z, Liang J, Shi W, Zhao Y, Luo Y, Ji X, Liu KJ (2015) Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp Neurol 272:181–189
Verkhratsky A, Petersen OH (2002) The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol 447:141–154
Xu F, Ma R, Zhang G, Wang S, Yin J, Wang E, Xiong E, Zhang Q, Li Y (2018) Estrogen and propofol combination therapy inhibits endoplasmic reticulum stress and remarkably attenuates cerebral ischemia-reperfusion injury and OGD injury in hippocampus. Biomed Pharmacother 108:1596–1606
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Marciniak SJ, Garcia-Bonilla L, Hu J, Harding HP, Ron D (2006) Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J Cell Biol 172:201–209
Remondelli P, Renna M (2017) The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front Mol Neurosci 10:187
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. Embo Rep 7:880–885
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Mengesdorf T, Proud CG, Mies G, Paschen W (2002) Mechanisms underlying suppression of protein synthesis induced by transient focal cerebral ischemia in mouse brain. Exp Neurol 177:538–546
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Nakka VP, Gusain A, Raghubir R (2010) Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res 17:189–202
Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M (2004) Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 11:403–415
Tuncay E, Bitirim VC, Durak A, Carrat G, Taylor KM, Rutter GA, Turan B (2017) Hyperglycemia-induced changes in ZIP7 and ZnT7 expression cause Zn(2+) release from the Sarco(endo)plasmic reticulum and mediate ER stress in the heart. Diabetes 66:1346–1358
Granzotto A, Canzoniero L, Sensi SL (2020) A neurotoxic menage-a-trois: glutamate, calcium, and zinc in the excitotoxic cascade. Front Mol Neurosci 13:60008
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10:780–791
Sinor JD, Du S, Venneti S, Blitzblau RC, Leszkiewicz DN, Rosenberg PA, Aizenman E (2000) NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro. J Neurosci 20:8831–8837
Koh JY, Kim HN, Hwang JJ, Kim YH, Park SE (2019) Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc. Mol Brain 12:18
Olgar Y, Durak A, Tuncay E, Bitirim CV, Ozcinar E, Inan MB, Tokcaer-Keskin Z, Akcali KC, Akar AR, Turan B (2018) Increased free Zn2+ correlates induction of sarco(endo)plasmic reticulum stressvia altered expression levels of Zn2+ -transporters in heart failure. J Cell Mol Med 22:1944–1956
Kawahara M, Sadakane Y, Koyama H, Konoha K, Ohkawara S (2013) d-Histidine and l-histidine attenuate zinc-induced neuronal death in GT1-7 cells. Metallomics 5:446–453
Kawahara M, Sadakane Y, Mizuno K, Kato-Negishi M, Tanaka KI (2020) Carnosine as a possible drug for zinc-induced neurotoxicity and vascular dementia. Int J Mol Sci 21:2570
Zhao Y, Fang Y, Zhao H, Li J, Duan Y, Shi W, Huang Y, Gao L, Luo Y (2018) Chrysophanol inhibits endoplasmic reticulum stress in cerebral ischemia and reperfusion mice. Eur J Pharmacol 818:1–9
Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK (2003) Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85:1026–1036
Wang X, Olberding KE, White C, Li C (2011) Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ 18:38–47
Mizuno D, Konoha-Mizuno K, Mori M, Sadakane Y, Koyama H, Ohkawara S, Kawahara M (2015) Protective activity of carnosine and anserine against zinc-induced neurotoxicity: a possible treatment for vascular dementia. Metallomics 7:1233–1239
Davis CK, Laud PJ, Bahor Z, Rajanikant GK, Majid A (2016) Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab 36:1686–1694
Tanaka K, Kawahara M (2017) Copper Enhances Zinc-Induced Neurotoxicity and the Endoplasmic Reticulum Stress Response in a Neuronal Model of Vascular Dementia. Front Neurosci-Switz 11
Tanaka KI, Kasai M, Shimoda M, Shimizu A, Kubota M, Kawahara M (2019) Nickel enhances zinc-induced neuronal cell death by priming the endoplasmic reticulum stress response. Oxid Med Cell Longev 2019:9693726
Doi T, Hara H, Kajita M, Kamiya T, Adachi T (2015) Zinc regulates expression of IL-23 p19 mRNA via activation of eIF2α/ATF4 axis in HAPI cells. Biometals 28:891–902
Alirezaei M, Nairn AC, Glowinski J, Premont J, Marin P (1999) Zinc inhibits protein synthesis in neurons. Potential role of phosphorylation of translation initiation factor-2alpha. J Biol Chem 274:32433–32438
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLOS Biol 4:e374
Germain M, Shore GC (2003) Cellular distribution of Bcl-2 family proteins. Sci STKE 2003:e10
Zhou W, Fang H, Wu Q, Wang X, Liu R, Li F, Xiao J, Yuan L, Zhou Z, Ma J, Wang L, Zhao W, You H, Ju J, Feng J, Chen C (2019) Ilamycin E, a natural product of marine actinomycete, inhibits triple-negative breast cancer partially through ER stress-CHOP-Bcl-2. Int J Biol Sci 15:1723–1732
Mann JJ, Fraker PJ (2005) Zinc pyrithione induces apoptosis and increases expression of Bim. Apoptosis 10:369–379
Iurlaro R, Munoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283:2640–2652
Acknowledgements
None.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.81971095 and No.81620108011).
Author information
Authors and Affiliations
Contributions
YZ designed the study. YZ, MD, NY, YH, CS and WS carried out the experiments and analyzed the data. YZ, MD, and WS contributed to figure design. YZ and MD wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Ding, M., Yang, N. et al. Zinc Accumulation Aggravates Cerebral Ischemia/Reperfusion Injury Through Inducing Endoplasmic Reticulum Stress. Neurochem Res 47, 1419–1428 (2022). https://doi.org/10.1007/s11064-022-03536-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03536-w