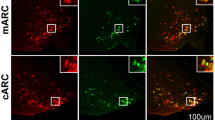
Abstract
The A11 region plays a role in numerous physiological functions, including pain and locomotor activity, and consists of a variety of neurons including GABAergic, calbindin positive (Calb+), and dopaminergic (DA) neurons. However, the neurochemical nature of Calb+ neurons and their regulatory role in the A11 region remain largely unknown. In this study, we examined the kind of functional markers co-expressed in the Calb+ neurons using sections from 8-week-old rats. To examine a marker related to classical neurotransmitters, we performed in situ hybridization for vesicular glutamate transporter 2 (vGluT2) or glutamate decarboxylase (GAD) 65 and 67, in conjunction with Calb immunohistochemistry. We found cellular co-expression of Calb with vGluT2 or GAD65/67 throughout the A11 region. Nearly all Calb+/GAD65/67+ neurons were found in the rostral-middle aspect of the A11 region. In contrast, Calb+/vGluT2+ neurons were found predominantly in the middle-caudal aspect of the A11 region. For receptors and neuropeptides, we performed immunohistochemistry for androgen receptor (AR), estrogen receptors (ERα and ERβ), and calcitonin gene-related peptide (CGRP). We found that Calb+ neurons co-expressed AR in the rostral aspect of the A11 region in both male and female rats. However, we rarely find cellular co-expression of Calb with ERα or ERβ in this region. For CGRP, we found both Calb+ neurons with or without CGRP expression. These results demonstrate that Calb+ neurons co-express many functional markers. Calb+ neurons have a distinct distribution pattern and may play a variety of regulatory roles, depending on their location within the A11 region.





Similar content being viewed by others
Data Availability
The data that support the findings of this study and materials used in this study are available from the corresponding author upon reasonable request.
References
Koblinger K, Jean-Xavier C, Sharma S, Füzesi T, Young L, Eaton SEA, Kwok CHT, Bains JS, Whelan PJ (2018) Optogenetic activation of A11 region increases motor activity. Front Neural Circuits 12:86
Charbit AR, Akerman S, Goadsby PJ (2011) Trigeminocervical complex responses after lesioning dopaminergic A11 nucleus are modified by dopamine and serotonin mechanisms. Pain 152:2365–2376
Charbit AR, Akerman S, Holland PR, Goadsby PJ (2009) Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci 29:12532–12541
Abdallah K, Monconduit L, Artola A, Luccarini P, Dallel R (2015) GABAAergic inhibition or dopamine denervation of the A11 hypothalamic nucleus induces trigeminal analgesia. Pain 156:644–655
Thorpe AJ, Clair A, Hochman S, Clemens S (2011) Possible sites of therapeutic action in restless legs syndrome: focus on dopamine and α2δ ligands. Eur Neurol 66:18–29
Guo CN, Yang WJ, Zhan SQ, Yang XF, Chen MC, Fuller PM, Lu J (2017) Targeted disruption of supraspinal motor circuitry reveals a distributed network underlying restless legs syndrome (RLS)-like movements in the rat. Sci Rep 7:9905–9919
Romero-Peralta S, Cano-Pumarega I, García-Borreguero D (2020) Emerging concepts of the pathophysiology and adverse outcomes of restless legs syndrome. Chest 158:1218–1229
Yamaguchi T, Sheen W, Morales M (2007) Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25:106–118
Yamaguchi T, Wang HL, Li X, Ng TH, Morales M (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31:8476–8490
Li X, Qi J, Yamaguchi T, Wang HL, Morales M (2013) Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct 218:1159–1176
Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci 41:760–772
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Omelchenko N, Sesack SR (2009) Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse 63:895–906
Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M (2010) Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci 30:218–229
Celio MR (1990) Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35:375–475
Duvarci S, Pare D (2014) Amygdala microcircuits controlling learned fear. Neuron 82:966–980
Olson VG, Nestler EJ (2007) Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse 61:87–95
Mongia S, Yamaguchi T, Liu B, Zhang S, Wang H, Morales M (2019) The ventral tegmental area has calbindin neurons with the capability to co-release glutamate and dopamine into the nucleus accumbens. Eur J Neurosci 50:3968–3984
Arai R, Jacobowitz DM, Deura S (1994) Distribution of calretinin, calbindin-D28k, and parvalbumin in the rat thalamus. Brain Res Bull 33:595–614
Frassoni C, Spreafico R, Bentivoglio M (1997) Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res 115:95–104
Ozawa H, Yamaguchi T, Hamaguchi S, Yamaguchi S, Ueda S (2017) Three types of A11 neurons project to the rat spinal cord. Neurochem Res 42:2142–2153
Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K (1987) Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 66:191–210
Yasui Y, Saper CB, Cechetto DF (1989) Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in the rat. J Comp Neurol 290:487–501
Yasui Y, Saper CB, Cechetto DF (1991) Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol 308:293–310
Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M (2005) Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol 489:92–119
Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95
Paxinos G, Watson C (2007) The raet brain in stereotaxic coordinates, 6th edn. Academic Press/Elsevier, Amsterdam/Boston
Pappas SS, Tiernan CT, Behrouz B, Jordan CL, Breedlove SM, Goudreau JL, Lookingland KJ (2010) Neonatal androgen-dependent sex differences in lumbar spinal cord dopamine concentrations and the number of A11 diencephalospinal dopamine neurons. J Comp Neurol 518:2423–2436
Pan PY, Ryan TA (2012) Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat Neurosci 15:813–815
van den Pol AN (1986) Tyrosine hydroxylase immunoreactive neurons throughout the hypothalamus receive glutamate decarboxylase immunoreactive synapses: a double pre-embedding immunocytochemical study with particulate silver and HRP. J Neurosci 6:877–891
Pappas SS, Kennedy T, Goudreau JL, Lookingland KJ (2011) Opioid-mediated regulation of A11 diencephalospinal dopamine neurons: pharmacological evidence of activation by morphine. Neuropharmacology 61:614–621
Moriizumi T, Hattori T (1992) Anatomical and functional compartmentalization of the subparafascicular thalamic nucleus in the rat. Exp Brain Res 90:175–179
Cortés R, Ceccatelli S, Schalling M, Hökfelt T (1990) Differential effects of intracerebroventricular colchicine administration on the expression of mRNAs for neuropeptides and neurotransmitter enzymes, with special emphasis on galanin: an in situ hybridization study. Synapse 6:369–391
Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR (1994) Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci 14:1834–1855
Heritage AS, Stumpf WE, Sar M, Grant LD (1980) Brainstem catecholamine neurons are target sites for sex steroid hormones. Science 207:1377–1379
Jahan MR, Kokubu K, Islam MN, Matsuo C, Yanai A, Wroblewski G, Fujinaga R, Shinoda K (2015) Species differences in androgen receptor expression in the medial preoptic and anterior hypothalamic areas of adult male and female rodents. Neuroscience 284:943–961
Sickel MJ, McCarthy MM (2000) Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol 12:397–402
Sá SI, Fonseca BM (2017) Dynamics of progesterone and estrogen receptor alpha in the ventromedial hypothalamus. J Endocrinol 233:197–207
Lonstein JS, Blaustein JD (2004) Immunocytochemical investigation of nuclear progestin receptor expression within dopaminergic neurones of the female rat brain. J Neuroendocrinol 16:534–543
McCutcheon JE, Marinelli M (2009) Age matters. Eur J Neurosci 29:997–1014
Acknowledgements
This work was supported by Dokkyo Medical University and by the Grants-in-Aid for Scientific Research (KAKENHI, Grant Number JP18K16495) from Japan Society for the Promotion of Science (JSPS). We would like to thank Ms. Shukuko Minami for her technical assistance and Ms. Fusae Terauchi for her assistance.
Funding
This study was funded by the Grants-in-Aid for Scientific Research (KAKENHI), Grant Number JP18K16495 from Japan Society for the Promotion of Science (JSPS) for HO.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Tsuyoshi Yamaguchi and Hidechika Ozawa. The first draft of the manuscript was written by Tsuyoshi Yamaguchi and Hidechika Ozawa, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Ozawa, H., Yamaguchi, S. et al. Calbindin-Positive Neurons Co-express Functional Markers in a Location-Dependent Manner Within the A11 Region of the Rat Brain. Neurochem Res 46, 853–865 (2021). https://doi.org/10.1007/s11064-020-03217-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03217-6