Abstract
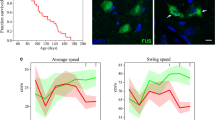
A number of mutations in a gene encoding RNA-binding protein FUS have been linked to the development of a familial form of amyotrophic lateral sclerosis known as FUS-ALS. C-terminal truncations of FUS by either nonsense or frameshift mutations lead to the development of FUS-ALS with a particularly early onset and fast progression. However, even in patients bearing these highly pathogenic mutations the function of motor neurons is not noticeably compromised for at least a couple of decades, suggesting that until cytoplasmic levels of FUS lacking its C-terminal nuclear localisation signal reaches a critical threshold, motor neurons are able to tolerate its permanent production. In order to identify how the nervous system responds to low levels of pathogenic variants of FUS we produced and characterised a mouse line, L-FUS[1–359], with a low neuronal expression level of a highly aggregation-prone and pathogenic form of C-terminally truncated FUS. In contrast to mice that express substantially higher level of the same FUS variant and develop severe early onset motor neuron pathology, L-FUS[1–359] mice do not develop any clinical or histopathological signs of motor neuron deficiency even at old age. Nevertheless, we detected substantial changes in the spinal cord transcriptome of these mice compared to their wild type littermates. We suggest that at least some of these changes reflect activation of cellular mechanisms compensating for the potentially damaging effect of pathogenic FUS production. Further studies of these mechanism might reveal effective targets for therapy of FUS-ALS and possibly, other forms of ALS.




Similar content being viewed by others
Data Availability
Data generated during this study are included in this published article and its Supplementary Information files and the RNA sequencing data were deposited in Gene Expression Omnibus (GEO) under the number GSE130604.
References
Deng HX, Zhai H, Bigio EH et al (2010) FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol 67(6):739–748. https://doi.org/10.1002/ana.22051
Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH (2012) The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol 124(3):339–352. https://doi.org/10.1007/s00401-012-1022-4
Polymenidou M, Lagier-Tourenne C, Hutt KR, Bennett CF, Cleveland DW, Yeo GW (2012) Misregulated RNA processing in amyotrophic lateral sclerosis. Brain Res 1462:3–15. https://doi.org/10.1016/j.brainres.2012.02.059
Deng H, Gao K, Jankovic J (2014) The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol 10(6):337–348. https://doi.org/10.1038/nrneurol.2014.78
Mackenzie IR, Munoz DG, Kusaka H, Yokota O, Ishihara K, Roeber S, Kretzschmar HA, Cairns NJ, Neumann M (2011) Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol 121(2):207–218. https://doi.org/10.1007/s00401-010-0764-0
Mackenzie IR, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9(10):995–1007. https://doi.org/10.1016/S1474-4422(10)70195-2
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR (2009) A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132(Pt 11):2922–2931. https://doi.org/10.1093/brain/awp214
Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR (2009) Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 118(5):605–616. https://doi.org/10.1007/s00401-009-0581-5
Snowden JS, Hu Q, Rollinson S (2011) The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene. Acta Neuropathol 122(1):99–110. https://doi.org/10.1007/s00401-011-0816-0
Nolan M, Talbot K, Ansorge O (2016) Pathogenesis of FUS-associated ALS and FTD: insights from rodent models. Acta Neuropathol Commun 4(1):99. https://doi.org/10.1186/s40478-016-0358-8
Van Damme P, Robberecht W, Van Den Bosch L (2017) Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis Models Mech 10(5):537–549. https://doi.org/10.1242/dmm.029058
Shang Y, Huang EJ (2016) Mechanisms of FUS mutations in familial amyotrophic lateral sclerosis. Brain Res 1647:65–78. https://doi.org/10.1016/j.brainres.2016.03.036
Shelkovnikova TA, Peters OM, Deykin AV et al (2013) Fused in Sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. J Biol Chem 288(35):25266–25274. https://doi.org/10.1074/jbc.M113.492017
DeJesus-Hernandez M, Kocerha J, Finch N (2010) De novo truncating FUS gene mutation as a cause of sporadic amyotrophic lateral sclerosis. Hum Mutat 31(5):E1377–1389. https://doi.org/10.1002/humu.21241
Bosco DA, Lemay N, Ko HK, Zhou H, Burke C, Kwiatkowski TJ Jr, Sapp P, McKenna-Yasek D, Brown RH Jr, Hayward LJ (2010) Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum Mol Genet 19(21):4160–4175. https://doi.org/10.1093/hmg/ddq335
Belzil VV, Langlais JS, Daoud H, Dion PA, Brais B, Rouleau GA (2012) Novel FUS deletion in a patient with juvenile amyotrophic lateral sclerosis. Arch Neurol 69(5):653–656. https://doi.org/10.1001/archneurol.2011.2499
Waibel S, Neumann M, Rosenbohm A, Birve A, Volk AE, Weishaupt JH, Meyer T, Müller U, Andersen PM, Ludolph AC (2013) Truncating mutations in FUS/TLS give rise to a more aggressive ALS-phenotype than missense mutations: a clinico-genetic study in Germany. Eur J Neurol 20(3):540–546. https://doi.org/10.1111/ene.12031
Lattante S, Rouleau GA, Kabashi E (2013) TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: Summary and update. Hum Mutat 34(6):812–826. https://doi.org/10.1002/humu.22319
Robinson HK, Deykin AV, Bronovitsky EV et al (2015) Early lethality and neuronal proteinopathy in mice expressing cytoplasm-targeted FUS that lacks the RNA recognition motif. Amyotroph Lateral Scler Frontotemporal Degener 16(5–6):402–409. https://doi.org/10.3109/21678421.2015.1040994
Ninkina N, Peters O, Millership S, Salem H, van der Putten H, Buchman VL (2009) Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein. Hum Mol Genet 18(10):1779–1794. https://doi.org/10.1093/hmg/ddp090
Connor-Robson N, Peters OM, Millership S, Ninkina N, Buchman VL (2016) Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behavior and dopamine metabolism. Neurobiol Aging 46:107–112. https://doi.org/10.1016/j.neurobiolaging.2016.06.020
Peters OM, Millership S, Shelkovnikova TA, Soto I, Keeling L, Hann A, Marsh-Armstrong N, Buchman VL, Ninkina N (2012) Selective pattern of motor system damage in gamma-synuclein transgenic mice mirrors the respective pathology in amyotrophic lateral sclerosis. Neurobiol Dis 48(1):124–131. https://doi.org/10.1016/j.nbd.2012.06.016
Millership S, Ninkina N, Guschina IA, Norton J, Brambilla R, Oort PJ, Adams SH, Dennis RJ, Voshol PJ, Rochford JJ, Buchman VL (2012) Increased lipolysis and altered lipid homeostasis protect gamma-synuclein-null mutant mice from diet-induced obesity. Proc Natl Acad Sci USA 109(51):20943–20948. https://doi.org/10.1073/pnas.1210022110
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1):15–21. https://doi.org/10.1093/bioinformatics/bts635
Krasnov GS, Dmitriev AA, Kudryavtseva AV et al (2015) PPLine: An automated pipeline for SNP, SAP, and splice variant detection in the context of proteogenomics. J Proteome Res 14(9):3729–3737. https://doi.org/10.1021/acs.jproteome.5b00490
Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30(7):923–930. https://doi.org/10.1093/bioinformatics/btt656
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. https://doi.org/10.1093/bioinformatics/btp616
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47. https://doi.org/10.1093/nar/gkv007
Bandyopadhyay U, Cotney J, Nagy M, Oh S, Leng J, Mahajan M, Mane S, Fenton WA, Noonan JP, Horwich AL (2013) RNA-Seq profiling of spinal cord motor neurons from a presymptomatic SOD1 ALS mouse. PLoS ONE 8(1):e53575. https://doi.org/10.1371/journal.pone.0053575
Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, Myers RM, Maniatis T (2013) A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4(2):385–401. https://doi.org/10.1016/j.celrep.2013.06.018
Funikov SY, Rezvykh AP, Mazin PV, Morozov AV, Maltsev AV, Chicheva MM, Vikhareva EA, Evgen'ev MB, Ustyugov AA (2018) FUS(1–359) transgenic mice as a model of ALS: pathophysiological and molecular aspects of the proteinopathy. Neurogenetics 19(3):189–204. https://doi.org/10.1007/s10048-018-0553-9
Fresno C, Fernandez EA (2013) RDAVIDWebService: a versatile R interface to DAVID. Bioinformatics 29(21):2810–2811. https://doi.org/10.1093/bioinformatics/btt487
Supek F, Bosnjak M, Skunca N, Smuc T (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6(7):e21800. https://doi.org/10.1371/journal.pone.0021800
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Walter W, Sanchez-Cabo F, Ricote M (2015) GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics 31(17):2912–2914. https://doi.org/10.1093/bioinformatics/btv300
Lopez-Erauskin J, Tadokoro T, Baughn MW et al (2018) ALS/FTD-linked mutation in FUS suppresses intra-axonal protein synthesis and drives disease without nuclear loss-of-function of FUS. Neuron 100(4):816–830. https://doi.org/10.1016/j.neuron.2018.09.044
Sharma A, Lyashchenko AK, Lu L, Nasrabady SE, Elmaleh M, Mendelsohn M, Nemes A, Tapia JC, Mentis GZ, Shneider NA (2016) ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat Commun 7:10465. https://doi.org/10.1038/ncomms10465
Devoy A, Kalmar B, Stewart M et al (2017) Humanized mutant FUS drives progressive motor neuron degeneration without aggregation in 'FUSDelta14' knockin mice. Brain 140(11):2797–2805. https://doi.org/10.1093/brain/awx248
Scekic-Zahirovic J, Sendscheid O, El Oussini H et al (2016) Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. EMBO J 35(10):1077–1097. https://doi.org/10.15252/embj.201592559
Shiihashi G, Ito D, Yagi T, Nihei Y, Ebine T, Suzuki N (2016) Mislocated FUS is sufficient for gain-of-toxic-function amyotrophic lateral sclerosis phenotypes in mice. Brain 139(Pt 9):2380–2394. https://doi.org/10.1093/brain/aww161
Humphrey J, Birsa N, Milioto C et al (2019) FUS ALS-causative mutations impact FUS autoregulation and the processing of RNA-binding proteins through intron retention. bioRxiv. https://doi.org/10.1101/567735
Scekic-Zahirovic J, Oussini HE, Mersmann S et al (2017) Motor neuron intrinsic and extrinsic mechanisms contribute to the pathogenesis of FUS-associated amyotrophic lateral sclerosis. Acta Neuropathol 133(6):887–906. https://doi.org/10.1007/s00401-017-1687-9
Lerga A, Hallier M, Delva L, Orvain C, Gallais I, Marie J, Moreau-Gachelin F (2001) Identification of an RNA binding specificity for the potential splicing factor TLS. J Biol Chem 276(9):6807–6816. https://doi.org/10.1074/jbc.M008304200
Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, Haass C (2012) Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J Biol Chem 287(27):23079–23094. https://doi.org/10.1074/jbc.M111.328757
Liu X, Niu C, Ren J, Zhang J, Xie X, Zhu H, Feng W (1832) Gong W (2013) The RRM domain of human fused in sarcoma protein reveals a non-canonical nucleic acid binding site. Biochim Biophys Acta 2:375–385. https://doi.org/10.1016/j.bbadis.2012.11.012
Shelkovnikova TA, Robinson HK, Connor-Robson N, Buchman VL (2013) Recruitment into stress granules prevents irreversible aggregation of FUS protein mislocalized to the cytoplasm. Cell Cycle 12(19):3194–3202. https://doi.org/10.4161/cc.26241
Shelkovnikova TA, Robinson HK, Southcombe JA, Ninkina N, Buchman VL (2014) Multistep process of FUS aggregation in the cell cytoplasm involves RNA-dependent and RNA-independent mechanisms. Hum Mol Genet 23(19):5211–5226. https://doi.org/10.1093/hmg/ddu243
Belly A, Moreau-Gachelin F, Sadoul R, Goldberg Y (2005) Delocalization of the multifunctional RNA splicing factor TLS/FUS in hippocampal neurones: exclusion from the nucleus and accumulation in dendritic granules and spine heads. Neurosci Lett 379(3):152–157. https://doi.org/10.1016/j.neulet.2004.12.071
Fujii R, Okabe S, Urushido T, Inoue K, Yoshimura A, Tachibana T, Nishikawa T, Hicks GG, Takumi T (2005) The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol 15(6):587–593. https://doi.org/10.1016/j.cub.2005.01.058
Schoen M, Reichel JM, Demestre M, Putz S, Deshpande D, Proepper C, Liebau S, Schmeisser MJ, Ludolph AC, Michaelis J, Boeckers TM (2015) Super-resolution microscopy reveals presynaptic localization of the ALS/FTD related protein FUS in Hippocampal neurons. Front Cell Neurosci 9:496. https://doi.org/10.3389/fncel.2015.00496
So E, Mitchell JC, Memmi C, Chennell G, Vizcay-Barrena G, Allison L, Shaw CE, Vance C (2018) Mitochondrial abnormalities and disruption of the neuromuscular junction precede the clinical phenotype and motor neuron loss in hFUSWT transgenic mice. Hum Mol Genet 27(3):463–474. https://doi.org/10.1093/hmg/ddx415
Udagawa T, Fujioka Y, Tanaka M et al (2015) FUS regulates AMPA receptor function and FTLD/ALS-associated behaviour via GluA1 mRNA stabilization. Nat Commun 6:7098. https://doi.org/10.1038/ncomms8098
Yasuda K, Zhang H, Loiselle D, Haystead T, Macara IG, Mili S (2013) The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules. J Cell Biol 203(5):737–746. https://doi.org/10.1083/jcb.201306058
Acknowledgements
We are thankful to Angela Marchbank and Georgina Smethurst from the Cardiff School of Biosciences Genomics Research Hub for help with RNA sequencing, Don Cleveland for sharing with us human FUS specific antibody, and Harri Harrison for critical reading of the manuscript. This study was supported by Russian Science Foundation Projects RScF#18–15-00357 (phenotyping of L-FUS mice), RScF#17-75-20-249, (producing the L-FUS mice) and the Motor Neuron Disease Association research grant (Buchman/Apr13/6096). Bioresource Collection of IPAC RAS (No. 0090-2017-0016) and Core Facility IGB RAS were used to maintain transgenic mice and test their behaviour using equipment of the Centre for Collective Use IPAC RAS. RNA sequencing analysis was supported by Russian President Foundation grant MК-3316.2019.4. Differential expression analysis was performed using the equipment of the Engelhardt Institute of Molecular Biology RAS “Genome” center (https://www.eimb.ru/rus/ckp/ccu_genome_c.php).
Author information
Authors and Affiliations
Contributions
NN and VLB conceived the project, designed the study, carried out certain experiments, interpreted experimental data and wrote the manuscript. EAL acquired and analysed molecular biology and behaviour data, prepared figures and wrote drafts of manuscript sections. SF and APR performed bioinformatic analysis of RNAseq data, drafted results and prepared corresponding table and figures. KDC and MSK performed immunohistochemical and Western blot analyses. AU and AVD produced transgenic mice and generated survival data. IMF and SB defined chromosomal localisation of transgenic cassettes in both hFUS[1–359] lines. SOB interpreted experimental data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (The Bioethics committee of Institute of Physiologically Active Compounds, Russian Academy of Sciences approval No. 20 dated 23.06.2017). All animal work was carried out in accordance with this approval and the Rules of Good Laboratory Practice in Russian Federation (2016).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lysikova, E.A., Funikov, S., Rezvykh, A.P. et al. Low Level of Expression of C-Terminally Truncated Human FUS Causes Extensive Changes in the Spinal Cord Transcriptome of Asymptomatic Transgenic Mice. Neurochem Res 45, 1168–1179 (2020). https://doi.org/10.1007/s11064-020-02999-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02999-z