Abstract
Previous studies have revealed that morin (MOR), a neuroactive bioflavonoid, with proven psychotropic and neuroprotective properties reduced schizophrenic-like behaviors in mice. This study further evaluated the ability of MOR to prevent and reverse ketamine-induced schizophrenic-like behaviors and the underlying neurochemical changes and increased oxidative/nitrergic stress in mice. In the preventive protocol, mice received intraperitoneal injection of MOR (100 mg/kg), reference antipsychotic drugs [haloperidol (1 mg/kg), risperidone (0.5 mg/kg)], or saline daily for 14 consecutive days prior to i.p. injection of ketamine (KET) (20 mg/kg/day) from the 8th to the 14th day. In the reversal protocol, the animals received KET or saline for 14 days prior to MOR, haloperidol, risperidone, or saline treatments. Schizophrenic-like behaviors: positive (open-field test), negative (social-interaction test) and cognitive (Y-maze test) symptoms were evaluated. Thereafter, the brain levels of dopamine, glutamate, 5-hydroxytryptamine and acetyl-cholinesterase, as well as biomarkers of oxidative/nitrergic stress were measured in the striatum, prefrontal-cortex (PFC) and hippocampus (HC). Morin prevented and reversed KET-induced hyperlocomotion, social and cognitive deficits. Also, MOR or risperidone attenuated altered dopaminergic, glutamatergic, 5-hydroxytryptaminergic and cholinergic neurotransmissions in brain region-dependent manner. The increased malondialdehyde and nitrite levels accompanied by decreased glutathione concentrations in the striatum, PFC and HC in KET-treated mice were significantly attenuated by MOR or risperidone. Taken together, these findings suggest that the anti-schizophrenic-like activity of MOR may be mediated via mechanisms related to attenuation of neurochemical changes and oxidative/nitrergic alterations in mice.










Similar content being viewed by others
References
Davis KL, Kahn RS, Ko G, Davidson M (1991) Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486
Chatterjee M, Rajkumar V, Surajit G, Gautam P (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 63:1161–1171
Monte AS, de Souza GC, Mclntyre RS, Joanna KS, dos Santos JV, Rafaela CC, Bruna MM, de Lucena RDF, Vasconcelos SMM, de Sousa FCF, Carvalho AF, Macêdo DS (2013) Prevention and reversal of ketamine-induced schizophrenia related behaviour by minocycline in mice: possible involvement of antioxidant and nitrergic pathway. J Psychopharmacol 27:1032–1043
Irifune M, Shimizu T, Nomoto M (1991) Ketamine-induced hyperlocomotion associated with alteration of presynaptic component of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav 40:399–407
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremer JD, Heninger GR, Bowers MB, Charney DS (1994) Subanesthetic effects of the noncompetetive NMDA antagonist ketamine in humans. Arch Gen Psychiatry 51:199–214
Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995) Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13:9–19
Krebs MO, Gauchy C, Desban M, Glowinski J, Kemel ML (1995) Role of dynorphin and GABA in the inhibitory regulation of NMDA-induced dopamine release in striosome- and matrix-enriched areas of the rat striatum. J Neurosci 14:2435–2443
Koh M, Shao T, Sherwood YA, Smith DR (2016) Impaired hippocampal-dependent memory and reduced parvalbumin-positive interneurons in a ketamine mouse model of schizophrenia. Schizophr Res 171:187–194
Harrison PJ (2000) Postmortem studies in schizophrenia. Dialogues Clin Neurosci 2:349–357
Chatterjee M, Seema S, Reena K, Verma AK, Gautam P (2012) Evaluation of the antipsychotic potential of Panax quinquefolium in ketamine induced experimental psychosis model in mice. Neurochem Res 37:759–770
Vasconcelos G, Naiara CX, Caren NS, de Sousa T, de Queiroz O, Laio LLL, de Lucena DF, Clarissa SG, Macêdo D, Silvânia M, Vasconcelo M (2015) Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: participation of antioxidant, nitrergic and neurotrophic mechanisms. Schizophr Res 165:163–170
Pazvantoglu O, Selek S, Okay IT (2009) Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci 63:693–700
Tsai MC, Liou CW, Lin TK (2013) Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatry Res 13:44–49
Gründer G, Hippius H, Carlsson A (2009) The ‘atypicality’ of antipsychotics: a concept reexamined and re-defined. Nat Rev Drug Discov 8:197–202
Linck VM, Bessa MM, Herrmann AP, Iwu MM, Okunji CO, Elisabetsky E (2012) 5-HT2A/C receptors mediate the antipsychotic-like effects of alstonine. Prog Neuro-Psychopharmacol Biol Psychiatry 36:29–33
Sreedharan V, Venkatachalam KK, Namasivayam N (2009) Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Investig New Drugs 27:21–30
Kapoor R, Kakkar P (2012) Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS ONE 7:416–463
Caselli A, Cirri P, Santi A, Paoli P (2016) Morin: a promising natural drug. Curr Med Chem 23:774–791
Yu ZF, Fong WP, Cheng CHK (2006) The dual actions of morin (3, 7, 2, 4-penta hydroxyl flavone) as a hypouricemic agent: uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther 316(5):169–175
Hyun HB, Lee WS, Go SI, Nagappan A, Park C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, Ryu CH, Shin SC, Choi YH (2015) The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol 46:2670–2678
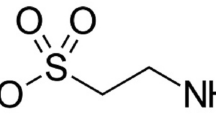
Merwid-Lad A, Trocha M, Chlebda E, Sozanski T, Magdalan J, Ksiadzyna D, Kopacz M, Kuzniar A, Nowak D, Piesniewska M, Fereniec-Golebiewska L, Kwiatkowska J, Szelag A (2012) Effects of morin-5′-sulfonic acid sodium salt (NaMSA) on cyclophosphamide-induced changes in oxidoredox state in rat liver and kidney. Hum Exp Toxicol 31:812–819
Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, Tatematsu N, Ushida J, Tsuda H, Mori H (1999) Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer 83:381–386
Sharma D, Singh M, Kumar P, Vikram V, Mishra N (2017) Development and characterization of morin hydrate loaded microemulsion for the management of Alzheimer’s disease. Artif Cells Nanomed Biotechnol 45:1620–1630
Gottlieb M, Rocı´o L, Marı´a RC, Marı´a VS, Elena A, Amaia A, Jose´ MD, Agne`s G, Carlos M (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis 23:374–386
Olonode ET, Aderibigbe AO, Adeoluwa OA, Eduviere AT, Ben-Azu B (2017) Morin hydrate mitigates rapid eye movement sleep deprivation-induced neurobehavioural impairments and loss of viable neurons in the hippocampus of mice. Behav Brain Res. https://doi.org/10.1016/j.bbr.2017.12.024
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Iwalewa EO (2017) Morin pretreatment attenuates schizophrenia-like behaviors in experimental animal models. Drug Res 68:159–167
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Umukoro S, Iwalewa EO (2018) Involvement of GABAergic, BDNF and Nox-2 mechanisms in the prevention and reversal of ketamine-induced schizophrenia-like behavior by morin in mice. Brain Res Bull 139:292–306
Omogbiya IA, Solomon U, Aderibigbe AO, Adewale GB (2013) Jobelyn pretreatment ameliorates symptoms of psychosis in experimental models. J Basic Clin Physiol Pharmacol 24:331–336
Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762:447–452
Jollow DJ, Michell JR, Zampaglione N, Gillete J (1974) Bromobenzene- induced liver necrosis. Protective role of glutathione an evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Okhawa H, Ohishi N, Yagi E (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germ free and conventional rat. Science 212:56–58
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by means of biuret reaction. J Biol Chem 177:751–766
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesteraseactivity. Biochem Pharmacol 7:88–95
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2) receptors—implications for models of schizophrenia. Mol Psychiatry 7:837–844
Lindefors N, Barati S, O’Connor WT (1997) Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res 759:205–212
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Verma A, Moghaddam B (1996) NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16:373–379
Ihalainen J, Savolainen K, Tanila H, Forsberg MM (2016) Comparison of phencyclidine-induced spatial learning and memory deficits and reversal by sertindole and risperidone between Lister hooded and Wistar rats. Behav Brain Res 305:140–147
Williams GV, Goldman-Racik PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575
Chatterjee M, Verma R, Kumari R, Singh S, Verma AK, Dwivedi AK, Gautam P (2015) Antipsychotic activity of standardized Bacopa extract against ketamine-induced experimental psychosis in mice: evidence for the involvement of dopaminergic, serotonergic, and cholinergic systems. Pharm Biol 53:1850–1860
Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y, Nitta A, Furukawa H, Nabeshima T (2007) Involvement of a dysfunctional sopamine-D1/ N-methyl-d-aspartate- NR1 and Ca2 þ/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol 71:1598–1609
Remya C, Dileep KV, Tintu I, Variyar EJ, Sadasivan C (2012) Design of potent inhibitors of acetylcholinesterase using morin as the starting compound. Front Life Sci 6:107–117
Paolucci E, Berretta N, Tozzi A, Bernardi G, Mercuri NB (2003) Depression of mGluR-mediated IPSCs by 5-HT in dopamine neurons of the rat substantia nigra pars compacta. Eur J Neurosci 18:2743–2750
Johnston A, Chris J, McBain Andre F (2014) 5-Hydroxytryptamine1A receptor-activation hyperpolarizespyramidal cells and suppresses hippocampal gammaoscillations via Kir3 channel activation. J Physiol 592:4187–4199
Meltzer HY (2010) Antipsychotic agents & Lithium. In: Katzung BG, Masters SB, Trevor AJ (eds) Basic & clinical pharmacology, 12th edn. McGraw-Hill Companies, New York
Meltzer HY, Horiguchi M, Massey BW (2011) The role of serotonin in the NMDAreceptor antagonist models of psychosis and cognitive impairment. Psychopharmacology 213:289–305
Onaolapo OJ, Ayanwale T, Agoi O, Adetimehin C, Onaolapo AY (2016) Zinc tempers haloperidol-induced behavioural changes in healthy mice. Int J Neurosci Behav Sci 4:21–31
Herrmann AP, Herrmann PL, Luísa KP, Tramontina AC, Viviane M, Linck COO, Carlos AG, Elisabetsky E (2012) Effects of the putative antipsychotic alstonine on glutamate uptake in acute hippocampal slices. Neurochem Int 61:1144–1150
Lisek M, Ferenc B, Studzian M, Pulaski L, Guo F, Zylinska L, Boczek T (2017) Glutamate deregulation in ketamine-induced psychosis—a potential role of PSD95, NMDA receptor and PMCA interaction. Front Cell Neurosci 11:181
Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ (2012) Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol Dis 47:338–346
Lisek M, Boczek T, Ferenc B, Zylinska L (2016) Regional brain dysregulation of Ca2C-handling systems in ketamine-induced rat model of experimental psychosis. Cell Tissue Res 363:609–620
Diamond JS (2001) Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci 21:8328–8338
Sleigh J, Harvey M, Voss L, Denny B (2014) Ketamine—more mechanisms of action than just NMDA blockade. Curr Anaesth Crit Care 4:76–81
Jia P, Wang L, Meltzer HY, Zhao Z (2010) Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res 122:38–42
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876
Baxter PS, Bell KFS, Hasel P, Kaindl AM, Fricker M, Thomson D, Cregan SP, Gillingwater TH, Hardingham GE (2015) Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6:6761
Ola MS, Aleisa AM, Al-Rejaie SS, Abuohashish HM, Parmar MY, Alhomida AS, Ahmed MM (2014) Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 35:1003–1008
Vanitha P, Uma C, Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, Gunasekaran P, Sivasubramanian S, Ramkumar KM (2014) Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotoc in induced diabetic rats. Environ Toxicol Pharmacol 37:326–335
Zhang Q, Zhang F, Thakur K, Wang J, Wang H, Hu F, Zhang JG, Wei ZJ (2018) Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem Toxicol 112:466–475
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Owoeye O, Olonode ET, Iwalewa EO (2018) Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother 105:1079–1090
West AR, Grace AA (2000) Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J Neurophysiol 83:1796–1808
Cheng A, Wang S, Cai J, Rao MS, Mattson MP (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258:319–333
Bernstein HG, Bogerts B, Keilhoff G (2005) The many faces of nitric oxide in schizophrenia: a review. Schizophr Res 78:69–86
Acknowledgements
The Authors are thankful to Dr. A. O. Odeseye and his colleagues of the Department of Microbiology and Biotechnology, Nigerian Institute of Science Laboratory Technology (NISLT) for his technical assistance during the enzyme immunoassay.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional (University of Ibadan Animal Care and Use Research Ethics Committee) guidelines for the care and use of animals were followed according to the ethical approval number: UI-ACUREC/App/12/2016/01.
Rights and permissions
About this article
Cite this article
Ben-Azu, B., Aderibigbe, A.O., Eneni, AE.O. et al. Morin Attenuates Neurochemical Changes and Increased Oxidative/Nitrergic Stress in Brains of Mice Exposed to Ketamine: Prevention and Reversal of Schizophrenia-Like Symptoms. Neurochem Res 43, 1745–1755 (2018). https://doi.org/10.1007/s11064-018-2590-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2590-z