Abstract
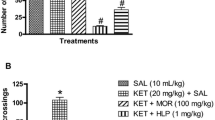
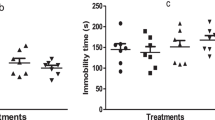
Mounting evidences have shown that nicotinamide adenine dinucleotide phosphate oxidase-2 (Nox-2) pathway modifies glutamic-acid decarboxylase-67 (GAD67) (GABAergic enzyme) and cholinergic systems via oxidative-nitrergic mechanisms in schizophrenia pathology. Rutin, a neuroactive antioxidant compound, with proven neuroprotective property has been shown to reduce schizophrenic-like behavior in mice. This study sought to investigate the mechanisms of action of the psychopharmacological activity of rutin in the preventive and reversal effects of ketamine-induced schizophrenic-like behavior, oxidative-nitrergic stress, cholinergic and GABAergic derangements in mice. In the preventive treatment, male mice were given rutin (0.1, 0.2 and 0.4 mg/kg) or risperidone (0.5 mg/kg) orally for 14 days prior to ketamine (20 mg/kg, i.p.) treatment from the 8 to 14th day. However, in the reversal treatment, ketamine was given for 14 days prior to rutin and risperidone. Behavioral (open-field, social-interaction and Y-maze tests), biochemical (oxidative/nitrergic stress markers, acetylcholinesterase activity), immunohistochemical (GAD67, Nox-2) and neuronal cell deaths in the striatum, prefrontal cortex, and hippocampus were evaluated. Ketamine-induced behavioral impairments were prevented and reversed by rutin. Exposure of mice to ketamine increased malondialdehyde, nitrite contents, acetylcholinesterase activity, neuronal cell death and Nox-2 expressions in the striatum, prefrontal cortex and hippocampus. Conversely, these derangements were prevented and reversed by rutin. The decreased glutathione levels due to ketamine were marked increased by rutin. Rutin only prevented ketamine-induced decrease in GAD67 expression in the striatal-hippocampal region. Altogether, the study showed that the prevention and reversal treatments of mice with rutin attenuated ketamine-induced schizophrenic-like behaviors via reduction of Nox-2 expression, oxidative/nitrergic stresses, acetylcholinesterase activity, and increased GAD67 enzyme.






Similar content being viewed by others
Abbreviations
- GAD67:
-
Glutamic-acid decarboxylase-67
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NMDA:
-
N-methyl-d-Aspartate
- KET:
-
Ketamine
- RUT:
-
Rutin
- SAB:
-
Spontaneous alternation behavior
- YMT:
-
Y-maze test
- SIT:
-
Social interaction test
- DTNB:
-
5′,5′-Dithio-bis-(2-nitrobenzoic acid)
- CA:
-
Cornu ammonis
- NO:
-
Nitric oxide
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide-dismutase
- AChE:
-
Acetyl-cholinesterase
- PFC:
-
Prefrontal cortex
- ST:
-
Striatum
- HC:
-
Hippocampus
References
Orrico-Sánchez A, López-Lacort M, Muñoz-Quiles C, Sanfélix-Gimeno G, Díez-Domingo J (2020) Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry 20:149
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA (2018) Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull 44:1195–1203
Larson MK, Walker EF, Compton MT (2010) Early signs, diagnosis and therapeutics of the prodromal phase of schizophrenia and related psychotic disorders. Expert Rev Neurother 10:1347–1359
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD (1994) Subanaesthetic effects of the non-competitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Chatterjee M, Rajkumar V, Surajit G, Gautam P (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 63:1161–1171
Ben-Azu B, Aderibigbe AO, Eneni AO, Ajayi AM, Umukoro S, Iwalewa EO (2018) Orin attenuates neurochemical changes and increased oxidative/nitrergic stress in brains of mice exposed to ketamine: prevention and reversal of schizophrenia-like symptoms. Neurochem Res 43:1745–1755
Fujihara K, Hideki M, Toshikazu K, Ryosuke K, Masahiko M, Chiyoko T, Nobuaki T, Yuchio Y (2015) Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology 40:2475–2486
Schmidt MJ, Mirnics K (2015) Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 40:190–206
Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC (2014) Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 8:230
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Umukoro S, Iwalewa EO (2018) Involvement of GABAergic, BDNF and Nox-2 mechanisms in the prevention and reversal of ketamine-induced schizophrenia-like behavior by morin in mice. Brain Res Bull 139:292–306
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11:2481–2504
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Omogbiya IA, Owoeye O, Umukoro S, Iwalewa EO (2019) Morin decreases cortical pyramidal neuron degeneration via inhibition of neuroinflammation in mouse model of schizophrenia. Int Immunopharmacol 70:338–435
Behrens MM, Ali SS, Laura LD (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28:13957–13966
Włodarczyk A, Szarmach J, Cubała WJ, Wiglusz MS (2017) Benzodiazepines in combination with antipsychotic drugs for schizophrenia: GABA-ergic targeted therapy. Psychiatr Danub 29:345–348
Nunes EA, Leila C, de Oliveira L, de Luca RD, João Q, Zugno A, Peregrino A, Alexandre J (2012) Effects of pregabalin on behavioral alterations induced by ketamine in rats. Rev Bras Psiquiatr 34:329–333
Kreft S, Knapp M, Kreft I (1997) Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J Agric Food Chem 47:4649–4652
Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A, Ashafaq M, Islam F, Siddiqui MS, Safhi MM, Islam F (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 17:340–352
Ahmed OM, Moneim AA, Yazid IA (2010) Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of ruta graveolens infusion and rutin in Nicotinamide streptozotocin-induced diabetic rat. Diabetol Croat 39:15–35
Webster RP, Gawde MD, Bhattacharya RK (1996) Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett 109:185–191
Panasiak W, Wleklik M, Oraczewska A, Luczak M (1989) Influence of flavonoids on combined experimental infections with EMC virus and Staphylococcus aureus in mice. Acta Microbiol Pol 38:185–188
Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, Marder M (2006) Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol 539:168–176
Nieoczym D, Socała K, Raszewski G, Wlaz P (2014) Effect of quercetin and rutin in some acute seizure models in mice. Prog Neuropsychopharmacol Biol Psychiatry 54:50–58
Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH (1999) Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol 51:519–526
Wang FM, Shing Y, Huen SY, Xue TH (2005) Neuroactive flavonoids interacting with GABAA receptor complex. Curr Drug Targets CNS Neurol Disord 4:575–585
Pandy V, Vijeepallam K (2017) Antipsychotic-like activity of scopoletin and rutin against the positive symptoms of schizophrenia in mouse models. Exp Anim 66:417–423
Bishnoi M, Chopra K, Kulkarni SK (2007) Protective effect of rutin, a polyphenolic flavonoid against haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Fundam Clin Pharmacol 21:521–529
Monte AS, de Souza GC, Mclntyre RS, Joanna KS, dos Santos JV, Rafaela CC, Bruna MM, de Lucena RDF (2013) Prevention and reversal of ketamine-induced schizophrenia related behaviour by minocycline in mice: possible involvement of antioxidant and nitrergic pathway. J Psychopharmacol 27:1032–1043
Jollow DJ, Michell JR, Zampaglione H, Gillete J (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione an evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Ohkawa H, Ohishi N, Yagi E (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ellman GL, Courtney KD, Andres V, Feather-Stone RM Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Owoeye O, Olonode T, Iwalewa EO (2018) Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother 105:1079–1090
Becker A, Grecksch G (2004) Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Prog Neuropsychopharmacol Biol Psychiatry 28:1267–1277
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther 128:419–432
Johnston A, Chris J, McBain AF (2014) 5-Hydroxytryptamine1A receptor-activation hyperpolarizespyramidal cells and suppresses hippocampal gammaoscillations via Kir3 channel activation. J Physiol 592:4187–4199
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L et al (2009) Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66:384–392
Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM (2006) A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 26:1604–1615
Alpak MG, Unal A, Ari M, Savas HA (2015) Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res 229(1–2):200–205
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130
Shinkai T, Ohmori O, Hori H, Nakamura J (2002) Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol Psychiatry 7:560–563
Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X (2016) Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol 771:84–92
Szwajgier D, Borowiec K, Zapp J (2020) Activity-guided isolation of cholinesterase inhibitors quercetin, rutin and kaempferol from Prunus persica fruit. Z Naturforsch 75:87–96
Ademosun AO, Oboh G, Bello F, Ayeni PO (2016) Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Complement Altern Med 21:11–17
Omar SH, Scott CJ, Hamlin AS, Obied HK (2018) Biophenols: enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 128:118–129
Vijayakumar S, Manogar P, Prabhu S, Singh RA (2018) Novel ligandbased docking; molecular dynamic simulations; and absorption, distribution, metabolism, and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer’s disease. J Pharm Anal 8:413–420
Yan X, Chen T, Zhang L, Du H (2018) Study of the interactions of forsythiaside and rutin with acetylcholinesterase (AChE). Int J Biol Macromol 119:1344–1352
Ou-yang Z, Cao X, Wei Y, Wei-Wan-Qi Z, Ming Z, Jin-ao D (2013) Pharmacokinetic study of rutin and quercetin in rats after oral administration of total flavones of mulberry leaf extract. Rev Bras Farmacogn 23:776–782
Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C (1997) Bioavailability of rutin and quercetin in rats. FEBS Lett 409:12–16
Acknowledgements
The Authors are grateful to Dr. Theophilus Jarikre of the Department of Veterinary Pathology, University of Ibadan for his technical assistance during the immunohistochemistry
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved and performed under the guidelines of University of Lagos’s Animals Ethic Committee (CMUL/HREC/01/19/481) and the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication number: 85–23, revised 1985).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oshodi, T.O., Ben-Azu, B., Ishola, I.O. et al. Molecular mechanisms involved in the prevention and reversal of ketamine-induced schizophrenia-like behavior by rutin: the role of glutamic acid decarboxylase isoform-67, cholinergic, Nox-2-oxidative stress pathways in mice. Mol Biol Rep 48, 2335–2350 (2021). https://doi.org/10.1007/s11033-021-06264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06264-6