Abstract
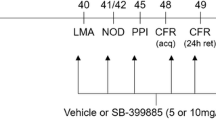
Schizophrenia is a debilitating disorder that may have a neurodevelopmental origin. For this reason, animal models based on neonatal insults or manipulations have been extensively used to demonstrate schizophrenia-related behaviors. Among those, the neonatal ventral hippocampus lesion (nVHL) is largely used as a model of schizophrenia-related behavior as it mimics behavioral and neurochemical abnormalities often seen in schizophrenic patients including hyperlocomotion in a novel environment. To investigate the neuroanatomical basis of coding novelty in the nVHL rat, we assessed the behavioral locomotor activity paradigm in a novel environment and measured expression of c-Fos, a marker of neural activation, in brain regions involved in the process of coding novelty or locomotion. Upon reaching adulthood, nVHL rats showed hyperlocomotion in the novel environment paradigm. Moreover, in nVHL rats the expression of c-Fos was greater in the prefrontal cortex (PFC) and CA1 region of the dorsal hippocampus compared to sham rats. Whereas similar expression of c-Fos was observed in the basolateral amygdala, nucleus accumbens and dentate gyrus region of hippocampus of nVHL and sham rats. These results suggest that the nVHL disrupts the neural activity in the PFC and CA1 region of hippocampus in the process of coding novelty in the rat.






Similar content being viewed by others
Change history
13 January 2018
The original version of this article unfortunately contained a mistake. The spelling of the author Tommaso Ianniti was incorrect and has been corrected as Tommaso Iannitti. The original article has been corrected.
References
Schmitt A, Malchow B, Hasan A, Falkai P (2014) The impact of environmental factors in severe psychiatric disorders. Front Neurosci 8:19. https://doi.org/10.3389/fnins.2014.00019
Flores G, Morales-Medina JC, Diaz A (2016) Neuronal and brain morphological changes in animal models of schizophrenia. Behav Brain Res 301:190–203. https://doi.org/10.1016/j.bbr.2015.12.034
Lipska BK, Weinberger DR (2000) To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 23(3):223–239. https://doi.org/10.1016/S0893-133X(00)00137-8
Alquicer G, Morales-Medina JC, Quirion R, Flores G (2008) Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J Chem Neuroanat 35(2):179–187. https://doi.org/10.1016/j.jchemneu.2007.10.001
Alquicer G, Silva-Gomez AB, Peralta F, Flores G (2004) Neonatal ventral hippocampus lesion alters the dopamine content in the limbic regions in postpubertal rats. Int J Dev Neurosci 22(2):103–111. https://doi.org/10.1016/j.ijdevneu.2003.12.003
Al-Amin HA, Shannon Weickert C, Weinberger DR, Lipska BK (2001) Delayed onset of enhanced MK-801-induced motor hyperactivity after neonatal lesions of the rat ventral hippocampus. Biol Psychiatry 49(6):528–539
Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK (1996) Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J Neurosci 16(22):7366–7375
Chambers RA, Moore J, McEvoy JP, Levin ED (1996) Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology 15(6):587–594. https://doi.org/10.1016/S0893-133X(96)00132-7
Sams-Dodd F, Lipska BK, Weinberger DR (1997) Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology 132(3):303–310
Save E, Poucet B, Foreman N, Buhot MC (1992) Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci 106(3):447–456
Mongiat LA, Esposito MS, Lombardi G, Schinder AF (2009) Reliable activation of immature neurons in the adult hippocampus. PloS ONE 4(4):e5320. https://doi.org/10.1371/journal.pone.0005320
Glausier JR, Lewis DA (2013) Dendritic spine pathology in schizophrenia. Neuroscience 251:90–107. https://doi.org/10.1016/j.neuroscience.2012.04.044
Hernandez A, Burton AC, O’Donnell P, Schoenbaum G, Roesch MR (2015) Altered basolateral amygdala encoding in an animal model of schizophrenia. J Neurosci 35(16):6394–6400. https://doi.org/10.1523/JNEUROSCI.5096-14.2015
Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK (2005) Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience 133(2):463–470. https://doi.org/10.1016/j.neuroscience.2005.02.021
Bringas ME, Morales-Medina JC, Flores-Vivaldo Y, Negrete-Diaz JV, Aguilar-Alonso P, Leon-Chavez BA, Lazcano-Ortiz Z, Monroy E, Rodriguez-Moreno A, Quirion R, Flores G (2012) Clozapine administration reverses behavioral, neuronal, and nitric oxide disturbances in the neonatal ventral hippocampus rat. Neuropharmacology 62(4):1848–1857. https://doi.org/10.1016/j.neuropharm.2011.12.008
Koleske AJ (2013) Molecular mechanisms of dendrite stability. Nat Rev Neurosci 14(8):536–550. https://doi.org/10.1038/nrn3486
Fiala JC, Spacek J, Harris KM (2002) Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev 39(1):29–54
Kovacs KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Intal 33(4):287–297
Flores G, Barbeau D, Quirion R, Srivastava LK (1996) Decreased binding of dopamine D3 receptors in limbic subregions after neonatal bilateral lesion of rat hippocampus. J Neurosci 16(6):2020–2026
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412
Silva-Gomez AB, Bermudez M, Quirion R, Srivastava LK, Picazo O, Flores G (2003) Comparative behavioral changes between male and female postpubertal rats following neonatal excitotoxic lesions of the ventral hippocampus. Brain Res 973(2):285–292
Juarez I, Silva-Gomez AB, Peralta F, Flores G (2003) Anoxia at birth induced hyperresponsiveness to amphetamine and stress in postpubertal rats. Brain Res 992(2):281–287
Dhakar MB, Rich ME, Reno EL, Lee HJ, Caldwell HK (2012) Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Horm Behav 62(1):86–92. https://doi.org/10.1016/j.yhbeh.2012.05.007
Paxinos G, Watson C (1997) The rat brainin stereotaxic coordinates, 3. Academic Press, San Diego
Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A (2009) Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol 12(8):1097–1110. https://doi.org/10.1017/S1461145709009985
Kumaran D, Maguire EA (2006) An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol 4(12):e424. https://doi.org/10.1371/journal.pbio.0040424
Kaplan R, Horner AJ, Bandettini PA, Doeller CF, Burgess N (2014) Human hippocampal processing of environmental novelty during spatial navigation. Hippocampus 24(7):740–750. https://doi.org/10.1002/hipo.22264
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J (2004) Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev 28(3):273–283. https://doi.org/10.1016/j.neubiorev.2004.03.004
Beer Z, Chwiesko C, Sauvage MM (2014) Processing of spatial and non-spatial information reveals functional homogeneity along the dorso-ventral axis of CA3, but not CA1. Neurobiol Learn Memory 111:56–64. https://doi.org/10.1016/j.nlm.2014.03.001
Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M (1999) Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol 38(2):234–246
Zhu XO, Brown MW, McCabe BJ, Aggleton JP (1995) Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience 69(3):821–829
Lee I, Hunsaker MR, Kesner RP (2005) The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci 119(1):145–153. https://doi.org/10.1037/0735-7044.119.1.145
Kabbaj M, Akil H (2001) Individual differences in novelty-seeking behavior in rats: a c-fos study. Neuroscience 106(3):535–545
Struthers WM, DuPriest A, Runyan J (2005) Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Exp Brain Ees 167(1):136–140. https://doi.org/10.1007/s00221-005-0061-7
Conde F, Maire-Lepoivre E, Audinat E, Crepel F (1995) Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol 352(4):567–593. https://doi.org/10.1002/cne.903520407
O’Donnell P, Lewis BL, Weinberger DR, Lipska BK (2002) Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex 12(9):975–982
French SJ, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119(1):19–31
Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63
Brady AM, Saul RD, Wiest MK (2010) Selective deficits in spatial working memory in the neonatal ventral hippocampal lesion rat model of schizophrenia. Neuropharmacology 59(7–8):605–611. https://doi.org/10.1016/j.neuropharm.2010.08.012
Fujise Y, Kubota M, Suzuki M, Tamano H, Takeda A (2017) Blockade of intracellular Zn2+ signaling in the basolateral amygdala affects object recognition memory via attenuation of dentate gyrus LTP. Neurochem Intal. https://doi.org/10.1016/j.neuint.2017.01.014
Acknowledgements
This study was supported by grants from Vicerrectoría de Investigación y Estudios de Posgrado (VIEP), Benemérita Universidad Autónoma de Puebla (BUAP) (No. 98-FLAG-2017) and Consejo Nacional de Ciencia y Tecnología (CONACYT) México (No. 252808) to GF. JCMM would like to thank the International Brain Research Organization (IBRO) for return home grant. We are grateful to Dr. Francisco Ramos Collazo for his help and suggestions related to animal care. TM and HTB acknowledge the CONACYT for studentships. RAVR, IMM, PAA, GF and JCMM thank the National Research System of Mexico for membership. We thank Mira Thakur for proofreading and editing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
The original version of this article was revised. The last name of the author Tommaso Ianniti had a spell error which has been corrected.
A correction to this article is available online at https://doi.org/10.1007/s11064-018-2468-0.
Rights and permissions
About this article
Cite this article
Monfil, T., Vázquez Roque, R.A., Camacho-Abrego, I. et al. Hyper-response to Novelty Increases c-Fos Expression in the Hippocampus and Prefrontal Cortex in a Rat Model of Schizophrenia. Neurochem Res 43, 441–448 (2018). https://doi.org/10.1007/s11064-017-2439-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2439-x