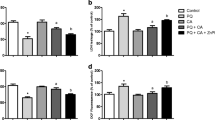
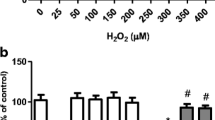
Abstract
Pinocembrin (PB; 5,7-dihydroxyflavanone) is found in propolis and exhibits antioxidant activity in several experimental models. The antioxidant capacity of PB is associated with the activation of the nuclear factor erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway. The Nrf2/ARE axis mediates the expression of antioxidant and detoxifying enzymes, such as glutathione peroxidase (GPx), glutathione reductase (GR), heme oxygenase-1 (HO-1), and the catalytic (GCLC) and regulatory (GCLM) subunits of the rate-limiting enzyme in the synthesis of glutathione (GSH), γ-glutamate-cysteine ligase (γ-GCL). Nonetheless, it is not clear how PB exerts mitochondrial protection in mammalian cells. Human neuroblastoma SH-SY5Y cells were pretreated (4 h) with PB (0–25 µM) and then exposed to methylglyoxal (MG; 500 µM) for further 24 h. Mitochondria were isolated by differential centrifugation. PB (25 µM) provided mitochondrial protection (decreased lipid peroxidation, protein carbonylation, and protein nitration in mitochondrial membranes; decreased mitochondrial free radical production; enhanced the content of GSH in mitochondria; rescued mitochondrial membrane potential—MMP) and blocked MG-triggered cell death by a mechanism dependent on the activation of the extracellular-related kinase (Erk1/2) and consequent upregulation of Nrf2. PB increased the levels of GPx, GR, HO-1, and mitochondrial GSH. The PB-induced effects were suppressed by silencing of Nrf2 with siRNA. Therefore, PB activated the Erk1/2–Nrf2 signaling pathway resulting in mitochondrial protection in SH-SY5Y cells exposed to MG. Our work shows that PB is a strong candidate to figure among mitochondria-focusing agents with pharmacological potential.















Similar content being viewed by others
References
Rasul A, Millimouno FM, Ali Eltayb W, Ali M, Li J, Li X (2013) Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed Res Int 2013:379850. doi:10.1155/2013/379850
Nina N, Quispe C, Jiménez-Aspee F, Theoduloz C, Feresín GE, Lima B, Leiva E, Schmeda-Hirschmann G (2015) Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, Central Chile. Molecules 20:18144–18167. doi:10.3390/molecules201018144
Zhou LT, Wang KJ, Li L, Li H, Geng M (2015) Pinocembrin inhibits lipopolysaccharide-induced inflammatory mediators production in BV2 microglial cells through suppression of PI3K/Akt/NF-κB pathway. Eur J Pharmacol 761:211–216. doi:10.1016/j.ejphar.2015.06.003
Promsan S, Jaikumkao K, Pongchaidecha A, Chattipakorn N, Chatsudthipong V, Arjinajarn P, Pompimon W, Lungkaphin A (2016) Pinocembrin attenuates gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol 94:808–818. doi:10.1139/cjpp-2015-0468
Liu R, Gao M, Yang ZH, Du GH (2008) Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia–reperfusion both in vivo and in vitro. Brain Res 1216:104–115. doi:10.1016/j.brainres.2008.03.049
Meng F, Liu R, Gao M, Wang Y, Yu X, Xuan Z, Sun J, Yang F, Wu C, Du G (2011) Pinocembrin attenuates blood–brain barrier injury induced by global cerebral ischemia–reperfusion in rats. Brain Res 1391:93–101. doi:10.1016/j.brainres.2011.03.010
Shi LL, Chen BN, Gao M, Zhang HA, Li YJ, Wang L, Du GH (2011) The characteristics of therapeutic effect of pinocembrin in transient global brain ischemia/reperfusion rats. Life Sci 88:521–528. doi:10.1016/j.lfs.2011.01.011
Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH (2013) Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 546:57–62. doi:10.1016/j.neulet.2013.04.060
Zhao G, Zhang W, Li L, Wu S, Du G (2014) Pinocembrin protects the brain against ischemia–reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules 19:15786–15798. doi:10.3390/molecules191015786
Liu R, Wu CX, Zhou D, Yang F, Tian S, Zhang L, Zhang TT, Du GH (2012) Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med 10:105. doi:10.1186/1741-7015-10-105
Liu R, Li JZ, Song JK, Sun JL, Li YJ, Zhou SB, Zhang TT, Du GH (2014) Pinocembrin protects human brain microvascular endothelial cells against fibrillar amyloid-β(1–40) injury by suppressing the MAPK/NF-κB inflammatory pathways. Biomed Res Int 2014:470393. doi:10.1155/2014/470393
Wang Y, Gao J, Miao Y, Cui Q, Zhao W, Zhang J, Wang H (2014) Pinocembrin protects SH-SY5Y cells against MPP+-induced neurotoxicity through the mitochondrial apoptotic pathway. J Mol Neurosci 53:537–545. doi:10.1007/s12031-013-0219-x
Gao M, Zhang WC, Liu QS, Hu JJ, Liu GT, Du GH (2008) Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of bax/bcl-2 ratio. Eur J Pharmacol 591:73–79. doi:10.1016/j.ejphar.2008.06.071
Jin X, Liu Q, Jia L, Li M, Wang X (2015) Pinocembrin attenuates 6-OHDA-induced neuronal cell death through Nrf2/ARE pathway in SH-SY5Y cells. Cell Mol Neurobiol 35:323–333. doi:10.1007/s10571-014-0128-8
Juurlink BH (1999) Management of oxidative stress in the CNS: the many roles of glutathione. Neurotox Res 1:119–140
Sorolla MA, Rodríguez-Colman MJ, Vall-llaura N, Tamarit J, Ros J, Cabiscol E (2012) Protein oxidation in Huntington disease. Biofactors 38:173–185
Sharma S, Moon CS, Khogali A, Haidous A, Chabenne A, Ojo C, Jelebinkov M, Kurdi Y, Ebadi M (2013) Biomarkers in Parkinson’s disease (recent update). Neurochem Int 63:201–229. doi:10.1016/j.neuint.2013.06.005
Taylor JM, Main BS, Crack PJ (2013) Neuroinflammation and oxidative stress: co-conspirators in the pathology of Parkinson’s disease. Neurochem Int 62:803–819. doi:10.1016/j.neuint.2012.12.016
Okon IS, Zou MH (2015) Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res 100:170–174. doi:10.1016/j.phrs.2015.06.013
Ali Sheikh MS, Salma U, Zhang B, Chen J, Zhuang J, Ping Z (2016) Diagnostic, prognostic, and therapeutic value of circulating miRNAs in heart failure patients associated with oxidative stress. Oxid Med Cell Longev 2016:5893064. doi:10.1155/2016/5893064
Bu J, Dou Y, Tian X, Wang Z, Chen G (2016) The role of omega-3 polyunsaturated fatty acids in stroke. Oxid Med Cell Longev 2016:6906712. doi:10.1155/2016/6906712
Kurian GA, Rajagopal R, Vedantham S, Rajesh M (2016) The Role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid Med Cell Longev 2016:1656450. doi:10.1155/2016/1656450
Lipchick BC, Fink EE, Nikiforov MA (2016) Oxidative stress and proteasome inhibitors in multiple myeloma. Pharmacol Res 105:210–215. doi:10.1016/j.phrs.2016.01.029
Vyas S, Zaganjor E, Haigis MC (2016) Mitochondria and cancer. Cell 166:555–566. doi:10.1016/j.cell.2016.07.002
Urano S, Sato Y, Otonari T, Makabe S, Suzuki S, Ogata M, Endo T (1998) Aging and oxidative stress in neurodegeneration. Biofactors 7:103–112
Forman HJ (2016) Redox signaling: an evolution from free radicals to aging. Free Radic Biol Med 97:398–407. doi:10.1016/j.freeradbiomed.2016.07.003
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Lynch RE, Fridovich I (1979) Autoinactivation of xanthine oxidase: the role of superoxide radical and hydrogen peroxide. Biochim Biophys Acta 571:195–200
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (2008) Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 283:21837–21841. doi:10.1074/jbc.R700019200
Fritz KS, Petersen DR (2011) Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem Res Toxicol 24:1411–1419. doi:10.1021/tx200169n
Guo J, Prokai-Tatrai K, Nguyen V, Rauniyar N, Ughy B, Prokai L (2011) Protein targets for carbonylation by 4-hydroxy-2-nonenal in rat liver mitochondria. J Proteom 74:2370–2379. doi:10.1016/j.jprot.2011.07.009
Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G (2008) Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53:126–131. doi:10.1016/j.neuint.2008.07.001
Abeti R, Abramov AY (2015) Mitochondrial Ca(2+) in neurodegenerative disorders. Pharmacol Res 99:377–381. doi:10.1016/j.phrs.2015.05.007
Angelova PR, Abramov AY (2016) Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2016.06.005. (in press).
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Liu Y, Song XD, Liu W, Zhang TY, Zuo J (2003) Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med 7:49–56
Yi F, He X, Wang D (2013) Lycopene protects against MPP(+)-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem Res 38:1747–1757. doi:10.1007/s11064-013-1079-z
Ye X, Han Y, Zhang L, Liu W, Zuo J (2015) MTERF4 regulates the mitochondrial dysfunction induced by MPP(+) in SH-SY5Y cells. Biochem Biophys Res Commun 464:214–220. doi:10.1016/j.bbrc.2015.06.119
Avetisyan AV, Samokhin AN, Alexandrova IY, Zinovkin RA, Simonyan RA, Bobkova NV (2016) Mitochondrial dysfunction in neocortex and hippocampus of olfactory bulbectomized mice, a model of Alzheimer’s disease. BioChemistry 81:615–623. doi:10.1134/S0006297916060080
Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G (2016) Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem 137:714–729. doi:10.1111/jnc.13590
Santa-Cruz LD, Guerrero-Castillo S, Uribe-Carvajal S, Tapia R (2016) Mitochondrial dysfunction during the early stages of excitotoxic spinal motor neuron degeneration in vivo. ACS Chem Neurosci 7:886–896. doi:10.1021/acschemneuro.6b00032
Tatarkova Z, Kovalska M, Timkova V, Racay P, Lehotsky J, Kaplan P (2016) The effect of aging on mitochondrial complex I and the extent of oxidative stress in the rat brain cortex. Neurochem Res 41:2160–2172. doi:10.1007/s11064-016-1931-z
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21:3017–3023
Sun AY, Draczynska-Lusiak B, Sun GY (2001) Oxidized lipoproteins, beta amyloid peptides and Alzheimer’s disease. Neurotox Res 3: 167–178
Calì T, Ottolini D, Brini M (2011) Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37:228–240
Keane PC, Kurzawa M, Blain PG, Morris CM (2011) Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis 2011: 716871. doi:10.4061/2011/716871
Guedes-Dias P, Pinho BR, Soares TR, de Proença J, Duchen MR, Oliveira JM (2016) Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol Dis 90:51–57. doi:10.1016/j.nbd.2015.09.008
Mejia EM, Chau S, Sparagna GC, Sipione S, Hatch GM (2016) Reduced mitochondrial function in human Huntington disease lymphoblasts is not due to alterations in cardiolipin metabolism or mitochondrial supercomplex assembly. Lipids 51:561–569. doi:10.1007/s11745-015-4110-0
Rochette L, Zeller M, Cottin Y, Vergely C (2014) Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840:2709–2729. doi:10.1016/j.bbagen.2014.05.017
Antoun G, McMurray F, Thrush AB, Patten DA, Peixoto AC, Slack RS, McPherson R, Dent R, Harper ME (2015) Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 58:2861–2866. doi:10.1007/s00125-015-3772-8
Wu F, Liu Y, Luo L, Lu Y, Yew DT, Xu J, Guo K (2015) Platelet mitochondrial dysfunction of DM rats and DM patients. Int J Clin Exp Med 8:6937–6946
Finsterer J, Ohnsorge P (2013) Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol 67:434–445. doi:10.1016/j.yrtph.2013.09.002
Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR (2015) Mitochondrial functional impairment in response to environmental toxins in the cardiorenal metabolic syndrome. Arch Toxicol 89:147–153. doi:10.1007/s00204-014-1431-3
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxid Med Cell Longev 2015:140267. doi:10.1155/2015/140267
Oliveira MR (2015) The neurotoxic effects of vitamin A and retinoids. Acad Bras Cienc 87:1361–1373. doi:10.1590/0001-3765201520140677
de Oliveira MR (2016) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. doi:10.1016/j.toxlet.2016.07.001
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. doi:10.1016/j.neuint.2015.12.006
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. doi:10.1016/j.biotechadv.2012.09.005
Gibellini L, Bianchini E, De Biasi S, Nasi M, Cossarizza A, Pinti M (2015) Natural compounds modulating mitochondrial functions. Evid Based Complement Altern Med 2015:527209. doi:10.1155/2015/527209
de Oliveira MR (2016) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. doi:10.1016/j.mito.2016.05.005
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016) Curcumin, mitochondrial biogenesis, and mitophagy: exploring recent data and indicating future needs. Biotechnol Adv 34:813–826. doi:10.1016/j.biotechadv.2016.04.004
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
Thornalley PJ, Wolff SP, Crabbe MJ, Stern A (1984) The oxidation of oxyhaemoglobin by glyceraldehyde and other simple monosaccharides. Biochem J 217:615–622
Roy SS, Biswas S, Ray M, Ray S (2003) Protective effect of creatine against inhibition by methylglyoxal of mitochondrial respiration of cardiac cells. Biochem J 372:661–669
Cardoso S, Carvalho C, Marinho R, Simões A, Sena CM, Matafome P, Santos MS, Seiça RM, Moreira PI (2014) Effects of methylglyoxal and pyridoxamine in rat brain mitochondria bioenergetics and oxidative status. J Bioenerg Biomembr 46:347–355. doi:10.1007/s10863-014-9551-2
Seo K, Seo S, Han JY, Ki SH, Shin SM (2014) Resveratrol attenuates methylglyoxal-induced mitochondrial dysfunction and apoptosis by Sestrin2 induction. Toxicol Appl Pharmacol 280:314–322. doi:10.1016/j.taap.2014.08.011
Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA 91:5710–5714
Harrington CR, Colaco CA (1994) Alzheimer’s disease. A glycation connection. Nature 370:247–248
Reddy VP, Obrenovich ME, Atwood CS, Perry G, Smith MA (2002) Involvement of Maillard reactions in Alzheimer disease. Neurotox Res. 4:191–209
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
Angeloni C, Malaguti M, Rizzo B, Barbalace MC, Fabbri D, Hrelia S (2015) Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem Res Toxicol 28:1234–1245. doi:10.1021/acs.chemrestox.5b00067
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. doi:10.1007/s10571-013-0006-9
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol. doi:10.1007/s12035-016-0009-x (in press)
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
de Oliveira MR, Moreira JC (2007) Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett 173:145–150
de Oliveira MR, Oliveira MW, Lorenzi R, Fagundes da Rocha R, Fonseca Moreira JC (2009) Short-term vitamin A supplementation at therapeutic doses induces a pro-oxidative state in the hepatic environment and facilitates calcium-ion-induced oxidative stress in rat liver mitochondria independently from permeability transition pore formation : detrimental effects of vitamin A supplementation on rat liver redox and bioenergetic states homeostasis. Cell Biol Toxicol 25:545–560. doi:10.1007/s10565-008-9111-9
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic acid affords mitochondrial protection in chlorpyrifos-treated Sh-Sy5y Cells. Neurotox Res. doi:10.1007/s12640-016-9620-x (in press)
de Oliveira MR, Lorenzi R, Schnorr CE, Morrone M, Moreira JC (2011) Increased 3-nitrotyrosine levels in mitochondrial membranes and impaired respiratory chain activity in brain regions of adult female rats submitted to daily vitamin A supplementation for 2 months. Brain Res Bull 86:246–253. doi:10.1016/j.brainresbull.2011.08.006
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Couto N, Wood J, Barber J (2016) The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 95:27–42. doi:10.1016/j.freeradbiomed.2016.02.028
Otterbein LE, Foresti R, Motterlini R (2016) Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and pro-survival. Circ Res 118:1940–1959. doi:10.1161/CIRCRESAHA.116.306588
Satoh T, McKercher SR, Lipton SA (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–657. doi:10.1016/j.freeradbiomed.2013.07.022
Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C (2014) NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79:298–306. doi:10.1016/j.neuropharm.2013.11.004
Matias I, Buosi AS, Gomes FC (2016) Functions of flavonoids in the central nervous system: astrocytes as targets for natural compounds. Neurochem Int 95:85–91. doi:10.1016/j.neuint.2016.01.009
Wang H, Wang Y, Zhao L, Cui Q, Wang Y, Du G (2016) Pinocembrin attenuates MPP(+)-induced neurotoxicity by the induction of heme oxygenase-1 through ERK1/2 pathway. Neurosci Lett 612:104–109. doi:10.1016/j.neulet.2015.11.048
Brekke E, Morken TS, Sonnewald U (2015) Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem Int 82:33–41. doi:10.1016/j.neuint.2015.02.002
Foresti R, Bains SK, Pitchumony TS, de Castro Brás LE, Drago F, Dubois-Randé JL, Bucolo C, Motterlini R (2013) Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res 76:132–148. doi:10.1016/j.phrs.2013.07.010
Jansen T, Daiber A (2012) Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front Pharmacol 3:30. doi:10.3389/fphar.2012.00030
Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL (2012) Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 32:1024–1034. doi:10.1038/jcbfm.2012.13
Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG (2005) Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289:H701–H707
Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J (2004) Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279:28681–28688
Stocker R, McDonagh AF, Glazer AN, Ames BN (1990) Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol 186:301–309
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq). GCF receives a “Bolsa Produtividade em Pesquisa”. GCF is supported by Edital APQ1/FAPERJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2016_2140_MOESM1_ESM.pdf
Supplementary material 1 Figure S1. A pretreatment with pinocembrin (PB) at 1–25 μM for 4 h ameliorates (a) cell viability and suppressed (b) cytotoxicity in human neuroblastoma SH-SY5Y cells exposed to methylglyoxal (MG) for additional 24 h. The results are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, #p < 0.05 vs the control group, *p < 0.05 different from MG-treated cells, **p < 0.01 different from MG-treated cells (PDF 92 KB)
11064_2016_2140_MOESM2_ESM.pdf
Supplementary material 2 Figure S2. Silencing of Nrf2 by using siRNA targeting Nrf2 in human neuroblastoma SH-SY5Y cells. Cells were further exposed to pinocembrin (PB) at 25 µM for 12 h. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 vs the control group, b p < 0.05 vs PB-treated cells transfected with negative control (NC) siRNA (PDF 102 KB)
11064_2016_2140_MOESM3_ESM.pdf
Supplementary material 3 Figure S3. The effects of a treatment with pinocembrin (25 µM) for different periods (0–24 h) on the cellular contents of a glutamate-cysteine ligase modifier subunit (GCLM), b glutamate-cysteine ligase catalytic subunit (GCLC), c glutathione peroxidase (GPx), and d glutathione reductase (GR) in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, ** p < 0.01 vs the control group (PDF 85 KB)
11064_2016_2140_MOESM4_ESM.pdf
Supplementary material 4 Figure S4. The effects of a treatment with pinocembrin (25 µM) for different periods (0–24 h) on the cellular contents of heme oxygenase-1 (HO-1) in human neuroblastoma SH-SY5Y cells. Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, **p < 0.01 vs the control group (PDF 99 KB)
11064_2016_2140_MOESM5_ESM.pdf
Supplementary material 5 Figure S5. The effects of a treatment with pinocembrin (PB) at varying concentrations (0–25 µM) for 12 h on the nuclear Nrf2 levels in human neuroblastoma SH-SY5Y cells (a). The time-dependent (0–12 h) effects of pinocembrin at 25 µM on the nuclear Nrf2 content (b). Data are presented as the mean ± SEM of three or five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, *p < 0.05 vs the control group, **p < 0.01 vs the control group (PDF 103 KB)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Peres, A. & Ferreira, G.C. Pinocembrin Attenuates Mitochondrial Dysfunction in Human Neuroblastoma SH-SY5Y Cells Exposed to Methylglyoxal: Role for the Erk1/2–Nrf2 Signaling Pathway. Neurochem Res 42, 1057–1072 (2017). https://doi.org/10.1007/s11064-016-2140-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2140-5