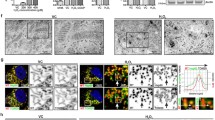
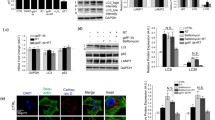
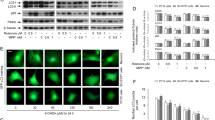
Abstract
Leucine-rich repeat kinase 2 (LRRK2) has been implicated in oxidative stress (OS) and neurodegeneration in Parkinson’s disease (PD). However, the pathophysiological mechanism of the LRRK2 kinase in neurons under stress stimuli is not yet understood. We demonstrate that rotenone (ROT), a mitochondria complex I inhibitor frequently used to generate in vitro and in vivo experimental models of PD, induces LRRK2 phosphorylation at serine 935 p-(S935) concomitant with cell death in nerve-like differentiated cells (NLCs). Indeed, ROT (50 µM) at 6 h exposure significantly increased reactive oxygen species (ROS) (~100 %), p-(S935)-LRRK2 kinase [~2 f(old)-(i)ncrease] level, induced nuclei condensation/fragmentation (16 %), increased the expression of NF-κB (5.6 f-i), p53 (5.3 f-i), c-Jun (5.4 f-i) transcription factors, activated caspase-3 (8.0 f-i) and AIF (6.8 f-i) proteins; but significantly decreased mitochondrial membrane potential (∆Ψm, ~21 %), indicative of apoptosis -a type of regulated cell death process- compared to untreated cells. Strikingly, the LRRK2 kinase inhibitor PF-06447475 (PF-475, 1 µM) protects NLCs against ROT induced noxious effect. The inhibitor not only blocked the p-(S935)-LRRK2 kinase phosphorylation but also completely abolished ROS, and significantly reversed all ROT-induced apoptosis signaling and OS associated markers to comparable control values. We conclude that wild-type LRRK2 may act as a pro-apoptotic factor under OS stimuli. Our findings suggest an association between OS and LRRK2 phosphorylation in the NLCs death process, as PD model. Therefore, the pharmacological inhibition of LRRK2 might help to understand the OS-mediated kinase activation in PD neurodegenerative disorder.












Similar content being viewed by others
References
Dickson DW (2012) Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. doi:10.1101/cshperspect.a009258
Spatola M, Wider C (2014) Genetics of Parkinson’s disease: the yield. Parkinsonism Relat Disord 20(Suppl 1):S35–S38
Goldman SM (2014) Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 54:141–164
Blesa J, Przedborski S (2014) Parkinson’s disease: animal models and dopaminergic cell vulnerability. Front Neuroanat 8:155
Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X, Lin Z, Wang T (2012) Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol 42:613–632
Johnson ME, Bobrovskaya L (2015) An update on the rotenone models of Parkinson’s disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology 46:101–116
Lambert AJ, Brand MD (2004) Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem 279:39414–39420
Tada-Oikawa S, Hiraku Y, Kawanishi M, Kawanishi S (2003) Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci 73:3277–3288
Michelini LG, Figueira TR, Siqueira-Santos ES, Castilho RF (2015) Rotenone exerts similar stimulatory effects on H2O2 production by isolated brain mitochondria from young-adult and old rats. Neurosci Lett 589:25–30
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525
Venderova K, Park DS (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med. doi:10.1101/cshperspect.a009365
Gaki GS, Papavassiliou AG (2014) Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular Med 16:217–230
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ 19:107–120
Avila-Gomez IC, Velez-Pardo C, Jimenez-Del-Rio M (2010) Effects of insulin-like growth factor-1 on rotenone-induced apoptosis in lymphocyte cells. Basic Clin Pharmacol Toxicol 106:53–61
Mendivil-Perez M, Jimenez-Del-Rio M, Velez-Pardo C (2014) Response to rotenone is glucose-sensitive in a model of human acute lymphoblastic leukemia: involvement of oxidative stress mechanism, DJ-1, Parkin, and PINK-1 proteins. Oxid Med Cell Longev 2014:457154
Gilsbach BK, Kortholt A (2014) Structural biology of the LRRK2 GTPase and kinase domains: implications for regulation. Front Mol Neurosci 7:32
Cruts M, Theuns J, Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat 33:1340–1344
Wallings R, Manzoni C, Bandopadhyay R (2015) Cellular processes associated with LRRK2 function and dysfunction. FEBS J 282:2806–2826
Esteves AR, Swerdlow RH, Cardoso SM (2014) LRRK2, a puzzling protein: insights into Parkinson’s disease pathogenesis. Exp Neurol 261:206–216
West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet 16:223–232
Heo HY, Park JM, Kim CH, Han BS, Kim KS, Seol W (2010) LRRK2 enhances oxidative stress-induced neurotoxicity via its kinase activity. Exp Cell Res 316:649–656
Li X, Moore DJ, Xiong Y, Dawson TM, Dawson VL (2010) Reevaluation of phosphorylation sites in the Parkinson disease-associated leucine-rich repeat kinase 2. J Biol Chem 285:29569–29576
Liou AK, Leak RK, Li L, Zigmond MJ (2008) Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis 32:116–124
Pereira C, Miguel Martins L, Saraiva L (2014) LRRK2, but not pathogenic mutants, protects against H2O2 stress depending on mitochondrial function and endocytosis in a yeast model. Biochim Biophys Acta 1840:2025–2031
Taghizadeh RR, Cetrulo KJ, Cetrulo CL (2011) Wharton’s jelly stem cells: future clinical applications. Placenta 32:S311–S315
Paldino E, Cenciarelli C, Giampaolo A, Milazzo L, Pescatori M, Hassan HJ, Casalbore P (2014) Induction of dopaminergic neurons from human Wharton’s jelly mesenchymal stem cell by forskolin. J Cell Physiol 229:232–244
Henderson JL, Kormos BL, Hayward MM, Coffman KJ, Jasti J, Kurumbail RG, Wager TT, Verhoest PR, Noell GS, Chen Y, Needle E, Berger Z, Steyn SJ, Houle C, Hirst WD, Galatsis P (2015) Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7 H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J Med Chem 58:419–432
Daher JP, Abdelmotilib HA, Hu X, Volpicelli-Daley LA, Moehle MS, Fraser KB, Needle E, Chen Y, Steyn SJ, Galatsis P, Hirst WD, West AB (2015) Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates α-Synuclein gene-induced neurodegeneration. J Biol Chem 290:19433–19444
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells, the international society for cellular therapy position statement. Cytotherapy 8:315–317
Hernandez DG, Paisán-Ruíz C, McInerney-Leo A, Jain S, Meyer-Lindenberg A, Evans EW, Berman KF, Johnson J, Auburger G, Schäffer AA, Lopez GJ, Nussbaum RL, Singleton AB (2005) Clinical and positron emission tomography of Parkinson’s disease caused by LRRK2. Ann Neurol 57:453–456
Huang S, Feng C, Wu Y, Yang S, Ma K, Wu X, Fu X (2013) Dissimilar characteristics of umbilical cord mesenchymal stem cells from donors of different ages. Cell Tissue Bank 14:707–713
Magavi SS, Macklis JD (2008) Immunocytochemical analysis of neuronal differentiation. Methods Mol Biol 438:345–352
Wlodkowic D, Skommer J, Darzynkiewicz Z (2009) Flow cytometry-based apoptosis detection. Methods Mol Biol 559:19–32
Arcila ML, Sánchez MD, Ortiz B, Barrera LF, García LF, Rojas M (2007) Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-alpha, IL-10, caspases and phospholipase A2. Cell Immunol 249:80–93
Siddiqui MA, Ahmad J, Farshori NN, Saquib Q, Jahan S, Kashyap MP, Ahamed M, Musarrat J, Al-Khedhairy AA (2013) Rotenone-induced oxidative stress and apoptosis in human liver HepG2 cells. Mol Cell Biochem 384(1–2):59–69
Watson N, Divers R, Kedar R, Mehindru A, Mehindru A, Borlongan MC, Borlongan CV (2015) Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy 17:18–24
Wyse RD, Dunbar GL, Rossignol J (2014) Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases. Int J Mol Sci 15:1719–1745
Subramanian A, Fong CY, Biswas A, Bongso A (2015) Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS One 10:e0127992
Kamikawaji S, Ito G, Iwatsubo T (2009) Identification of the autophosphorylation sites of LRRK2. BioChemistry 48:10963–10975
Reczek CR, Chandel NS (2015) ROS-dependent signal transduction. Curr Opin Cell Biol 33:8–13
Marinho HS, Real C, Cyrne L, Soares H, Antunes F (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2:535–562
Gaestel M (2016) MAPK-activated protein kinases (MKs): novel insights and challenges. Front cell. Dev Biol 3:88
Dzamko N, Inesta-Vaquera F, Zhang J, Xie C, Cai H, Arthur S, Tan L, Choi H, Gray N, Cohen P, Pedrioli P, Clark K, Alessi DR (2012) The IkappaB kinase family phosphorylates the Parkinson’s disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling. PLoS One 7:e39132
Chen CY, Weng YH, Chien KY, Lin KJ, Yeh TH, Cheng YP, Lu CS, Wang HL (2012) (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Diff 19:1623–1633
Angeles DC, Gan BH, Onstead L, Zhao Y, Lim KL, Dachsel J, Melrose H, Farrer M, Wszolek ZK, Dickson DW, Tan EK (2011) Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum Mutat 32:1390–1397
Niu J, Yu M, Wang C, Xu Z (2012) Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via dynamin-like protein. J Neurochem 122:650–658
Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X (2012) LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet 21:1931–1944
Yang D, Li T, Liu Z, Arbez N, Yan J, Moran TH, Ross CA, Smith WW (2012) LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a drosophila model: suppression by curcumin. Neurobiol Dis 47:385–392
Mamais A, Chia R, Beilina A, Hauser DN, Hall C, Lewis PA, Cookson MR, Bandopadhyay R (2014) Arsenite stress down-regulates phosphorylation and 14-3-3 binding of leucine-rich repeat kinase 2 (LRRK2), promoting self-association and cellular redistribution. J Biol Chem 289:21386–21400
Jimenez-Del-Rio M, Velez-Pardo C (2012) The bad, the good and the ugly about oxidative stress. Oxid Med Cellular Long 2012:1–13
Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, Yao A, Niu J, Zhang W, Evans DB, Abbruzzese JL, Huang P, Chiao PJ (2004) Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem 279:27549–27559
Mendivil-Perez M, Velez-Pardo C, Jimenez-Del-Rio M (2015) Doxorubicin induces apoptosis in Jurkat cells by mitochondria-dependent and mitochondria-independent mechanisms under normoxic and hypoxic conditions. Anticancer Drugs 26:583–598
Borner C, Andrews DW (2014) The apoptotic pore on mitochondria: are we breaking through or still stuck? Cell Death Differ 21:187–191
Sevrioukova IF (2011) Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal 14:2545–2579
Yao C, Johnson WM, Gao Y, Wang W, Zhang J, Deak M, Alessi DR, Zhu X, Mieyal JJ, Roder H, Wilson-Delfosse AL, Chen SG (2013) Kinase inhibitors arrest neurodegeneration in cell and C. elegans models of LRRK2 toxicity. Hum Mol Genet 22:328–344
Kramer T, Lo Monte F, Göring S, Okala Amombo GM, Schmidt B (2012) Small molecule kinase inhibitors for LRRK2 and their application to Parkinson’s disease models. ACS Chem Neurosci 3:151–160
Wang D, Tang B, Zhao G, Pan Q, Xia K, Bodmer R, Zhang Z (2008) Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol Neurodegener 3:3
Dzamko N, Chua G, Ranola M, Rowe DB, Halliday GM (2013) Measurement of LRRK2 and Ser910/935 phosphorylated LRRK2 in peripheral blood mononuclear cells from idiopathic Parkinson’s disease patients. J Parkinsons Dis 3:145–152
Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT (2004) Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci 117 (Pt 7):1201–1210
Acknowledgments
This study was supported by Colciencias grants #1115-545-31420 (contract 391) to MJ-Del-Rio and CV-P. MM-P is a Doctoral student at the “Corporacion Ciencias Basicas Biomedicas” program (CCBB)-University of Antioquia-UdeA. MM-P is funded by Colciencias Grants 567–2012. The use and technical assistance of the Flow Cytometry Unit of GICIG-SIU-UdeA and use of Odyssey Infrared Imaging System (Neuroscience Research Group, UdeA) are acknowledged.
Author Contributions
MM-P designed and performed experiments, analyzed data; CV-P and MJ-R obtained funding, designed experiments, provided reagents, analyzed data, critical reading of the manuscript and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Rights and permissions
About this article
Cite this article
Mendivil-Perez, M., Velez-Pardo, C. & Jimenez-Del-Rio, M. Neuroprotective Effect of the LRRK2 Kinase Inhibitor PF-06447475 in Human Nerve-Like Differentiated Cells Exposed to Oxidative Stress Stimuli: Implications for Parkinson’s Disease. Neurochem Res 41, 2675–2692 (2016). https://doi.org/10.1007/s11064-016-1982-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1982-1