Abstract
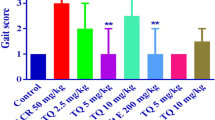
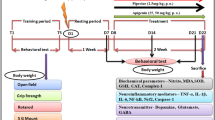
Organophosphate-induced delayed neuropathy (OPIDN) is pathologically characterized by the swollen axon containing aggregations of microtubules, neurofilaments, smooth endoplasmic reticulum and multivesicular vesicles. At present, the exact mechanism of OPIDN is unclear and the effective therapeutic methods is not available to counter this syndrome. Recent studies had shown that the autophagy was involved in OPIDN. The adipocytokine Apelin is a peptide, Apelin and its receptor are abundantly expressed in the nervous system. Recent researches illuminated that Apelin was neuroprotective factor and Apelin could regulate the autophagy in vivo and vitro model. So we investigated the effect of Apelin-13 on the OPIDN induced by Tri-ortho-cresyl phosphate (TOCP) in hens and explored the role of autophagy in Apelin-13 preventing OPIDN. Adult Roman hens were given a single dose of 750 mg/kg TOCP by gavage for 21 days to induce OPIDN, and neural dysfunction were detected, and the formation of autophagosomes in spinal cord neurons was observed by transmission electron microscopy, and the molecular markers of autophagy microtubule-associated protein light chain-3 (LC3) and the autophagy substrates p62/SQSTM1 were determined by Western blot analysis. The results demonstrated that the obvious neurological dysfunction such as hindlimb paralysis and paralysis of gait was present, the number of autophagosomes in the neurons of spinal cords was significantly increased, the level of LC3-II and p62 expressions and the ratio of LC3-II/LC3-I in spinal cords and sciatic nerve were significantly increased in the OPIDN model group compared with the control group. Compared with the OPIDN model group, the neurological dysfunction of tens was obviously reduced, the clinical signs scores was significantly decreased, the number of autophagosomes in the neurons of hen spinal cords was significantly decreased, the level of LC3-II and p62 expressions and the ratio of LC3-II/LC3-I in spinal cords and sciatic nerve were significantly decreased in Apelin-13 treatment group. Our results suggested that Apelin-13 prevented against the OPIDN induced by TOCP in hens, which the mechanism might be associated with regulation autophagy flux by Apelin-13.




Similar content being viewed by others
References
Gao X, Lin H, Ray R, Ray P (2013) Toxicogenomic studies of human neural cells following exposure to organophosphorus chemical warfare nerve agent VX. Neurochem Res 38(5):916–934
Jiang Y, Liu X, Li S, Zhang Y, Piao F, Sun X (2013) Identification of differentially expressed proteins related to organophosphorus-induced delayed neuropathy in the brains of hens. J Appl Toxicol 34(12):1352–1360
Song F, Xie K (2012) Calcium-dependent neutral cysteine protease and organophosphate-induced delayed neuropathy. Chem Biol Interact 200(2–3):114–118
Jokanović M, Kosanović M, Brkić D, Vukomanović P (2011) Organophosphate induced delayed polyneuropathy in man: an overview. Clin Neurol Neurosurg 113(1):7–10
Lotti M, Moretto A (2005) Organophosphate-induced delayed polyneuropathy. Toxicol Rev 24(1):37–49
Masoud A, Sandhir R (2012) Increased oxidative stress is associated with the development of organophosphate-induced delayed neuropathy. Hum Exp Toxicol 31(12):1214–1227
Masoud A, Kiran R, Sandhir R (2009) Impaired mitochondrial functions in organophosphate induced delayed neuropathy in rats. Cell Mol Neurobiol 29(8):1245–1255
Song F, Yan Y, Zhao X, Dou D, Zhang C, Xie K (2009) Phenylmethylsulfonyl fluoride protects against the degradation of neurofilaments in tri-ortho-cresyl phosphate (TOCP) induced delayed neuropathy. Toxicology 262(3):258–264
Gambalunga A, Pasqualato F, Lotti M (2010) Soluble phenyl valerate esterases of hen sciatic nerve and the potentiation of organophosphate induced delayed polyneuropathy. Chem Biol Interact 187(1–3):340–343
Hou WY, Long DX, Wang HP, Wang Q, Wu YJ (2008) The homeostasis of phosphatidylcholine and lysophosphatidylcholine was not disrupted during tri-o-cresyl phosphate-induced delayed neurotoxicity in hens. Toxicology 252(1–3):56–63
Moretto A, Nicolli A, Lotti M (2005) Peripheral nerve esterases and the promotion of organophosphate-induced neuropathy in hens. Chem Biol Interact 157–158:285–291
Jortner BS (2000) Mechanisms of toxic injury in the peripheral nervous system: neuropathologic considerations. Toxicol Pathol 28(1):54–69
Jortner BS (2011) Preparation and analysis of the peripheral nervous system. Toxicol Pathol 39(1):66–72
Emerick GL, Peccinini RG, de Oliveira GH (2009) Organophosphorus-induced delayed neuropathy: a simple and efficient therapeutic strategy. Toxicol Lett 192(2):238–244
Su J, Zhang T, Wang K, Zhu T, Li X (2014) Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochem Res 39(11):2068–2077
He Y, Zhao X, Subahan NR, Fan L, Gao J, Chen H (2014) The prognostic value of autophagy-related markers beclin-1 and microtubule-associated protein light chain 3B in cancers: a systematic review and meta-analysis. Tumour Biol 35(8):7317–7326
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873
Song JX, Lu JH, Liu LF, Chen LL, Durairajan SS, Yue Z, Zhang HQ, Li M (2014) HMGB1 is involved in autophagy inhibition caused by SNCA/α-synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy 10(1):144–1454
Dancourt J, Melia TJ (2014) Lipidation of the autophagy proteins LC3 and GABARAP is a membrane-curvature dependent process. Autophagy 10(8):1470–1471
Song F, Kou R, Zou C, Gao Y, Zeng T, Xie K (2014) Involvement of autophagy in tri-ortho-cresyl phosphate-induced delayed neuropathy in hens. Neurochem Int 64:1–8
Song F, Han X, Zeng T, Zhang C, Zou C, Xie K (2012) Changes in beclin-1 and micro-calpain expression in tri-ortho-cresyl phosphate-induced delayed neuropathy. Toxicol Lett 210(3):276–284
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251(2):471–476
O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T (1993) A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136(1–2):355–360
Rayalam S, Della-Fera MA, Kasser T, Warren W, Baile CA (2011) Emerging role of apelin as a therapeutic target in cancer: a patent review. Recent Pat Anticancer Drug Discov 6(3):367–372
Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P (2011) Apelin, diabetes, and obesity. Endocrine 40(1):1–9
Bodineau L, Taveau C, Lê Quan Sang HH, Osterstock G, Queguiner I, Moos F, Frugière A, Llorens-Cortes C (2011) Data supporting a new physiological role for brain apelin in the regulation of hypothalamic oxytocin neurons in lactating rats. Endocrinology 152(9):3492–3503
Zhang X, Peng X, Fang M, Zhou C, Zhao F, Zhang Y, Xu Y, Zhu Q, Luo J, Chen G, Wang X (2011) Up-regulation of apelin in brain tissue of patients with epilepsy and an epileptic rat model. Peptides 32(9):1793–1799
Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen J, Bai B (2014) Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides 63C:55–62
Zeng XJ, Yu SP, Zhang L, Wei L (2010) Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res 316(11):1773–1783
Zhang H, Gong Y, Wang Z, Jiang L, Chen R, Fan X, Zhu H, Han L, Li X, Xiao J, Kong X (2014) Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med 18(3):542–553
Bao HJ, Zhang L, Han WC, Dai DK (2015) Apelin-13 attenuates traumatic brain injury-induced damage by suppressing autophagy. Neurochem Res 40(1):89–97
Zou C, Kou R, Gao Y, Xie K, Song F (2013) Activation of mitochondria-mediated apoptotic pathway in tri-ortho-cresyl phosphate-induced delayed neuropathy. Neurochem Int 62(7):965–972
Honorato de Oliveira G, Moreira V, Ribeiro Goes SP (2002) Organophosphate induced delayed neuropathy in genetically dissimilar chickens: studies with tri-ortho-cresyl phosphate (TOCP) and trichlorfon. Toxicol Lett 136(2):143–150
Masoud A, Sandhir R (2012) Increased oxidative stress is associated with the development of organophosphate-induced delayed neuropathy. Hum Exp Toxicol 31(12):1214–1227
Makhaeva GF, Malygin VV, Strakhova NN, Sigolaeva LV, Sokolovskaya LG, Eremenko AV, Kurochkin IN, Richardson RJ (2007) Biosensor assay of neuropathy target esterase in whole blood as a new approach to OPIDN risk assessment: review of progress. Hum Exp Toxicol 26(4):273–282
Song F, Yan Y, Zhao X, Zhang C, Xie K (2009) Neurofilaments degradation as an early molecular event in tri-ortho-cresyl phosphate (TOCP) induced delayed neuropathy. Toxicology 258(2–3):94–100
Zhao XL, Zhang TL, Zhang CL, Han XY, Yu SF, Li SX, Cui N, Xie KQ (2006) Expression changes of neurofilament subunits in the central nervous system of hens treated with tri-ortho-cresyl phosphate (TOCP). Toxicology 223(1–2):127–135
Honorato de Oliveira G, Moreira V, Ribeiro Goes SP (2002) Organophosphate induced delayed neuropathy in genetically dissimilar chickens: studies with tri-ortho-cresyl phosphate (TOCP) and trichlorfon. Toxicol Lett 136(2):143–150
Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC (2007) Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol 102(6):518–528
O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL (2007) Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem 102(6):1905–1917
Masoud A, Sandhir R (2012) Increased oxidative stress is associated with the development of organophosphate-induced delayed neuropathy. Hum Exp Toxicol 31(12):1214–1227
Masoud A, Kiran R, Sandhir R (2011) Modulation of dopaminergic system and neurobehavioral functions in delayed neuropathy induced by organophosphates. Toxicol Mech Methods 21(1):1–5
Chen A, Xiong LJ, Tong Y, Mao M (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep 8(4):1011–1016
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6(3):366–377
Fu MM, Holzbaur EL (2014) MAPK8IP1/JIP1 regulates the trafficking of autophagosomes in neurons. Autophagy 10(11):2079–2081
Yoon SY, Choi JE, Kweon HS, Choe H, Kim SW, Hwang O, Lee H, Lee JY, Kim DH (2008) Okadaic acid increases autophagosomes in rat neurons: implications for Alzheimer’s disease. J Neurosci Res 86(14):3230–3239
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19(8):983–997
Son SM, Song H, Byun J, Park KS, Jang HC, Park YJ, Mook-Jung I (2012) Accumulation of autophagosomes contributes to enhanced amyloidogenic APP processing under insulin-resistant conditions. Autophagy 8(12):1842–1844
Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445:77–88
Giménez-Xavier P, Francisco R, Platini F, Pérez R, Ambrosio S (2008) LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int J Mol Med 22(6):781–785
Huang AC, Lien JC, Lin MW, Yang JS, Wu PP, Chang SJ, Lai TY (2013) Tetrandrine induces cell death in SAS human oral cancer cells through caspase activation-dependent apoptosis and LC3-I and LC3-II activation-dependent autophagy. Int J Oncol 43(2):485–494
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282(33):24131–24145
Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, Campello S, Nardacci R, Piacentini M, Campanella M, Cecconi F (2015) AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ 22(3):419–432
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM (2014) Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10(12):2208–2222
Jiang P, Mizushima N (2015) LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75:13–18
Acknowledgments
The work was supported by the construct program of the key discipline in hunan province, China (Basic Medicine Sciences in University of South China), Zhengxiang Scholar Program of University of South China (2014-004) and the National Natural Science Foundation of China (Grant No. 81171281).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
The manuscript does not contain clinical studies or patient data. Standards of animal care were in accordance with the ARRIVE guidelines and China law.
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s11064-016-2049-z.
The article has been retracted by the Editor-in-Chief due to the fact that Figure 2 was copied from the earlier publication by Song et al., Involvement of autophagy in tri-ortho-cresyl phosphate-induced delayed neuropathy in hens, Neurochem. Int. (2014) 64:1-8 (DOI: 10.1016/j.neuint.2013.10.017 ). The figure does not reflect the experiment described. The corresponding author has acknowledged this and agrees to the retraction.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11064-016-2049-z.
About this article
Cite this article
Zhou, Sh., Ouyang, Xp., Tian, Sw. et al. RETRACTED ARTICLE: Apelin-13 Prevents the Delayed Neuropathy Induced by Tri-ortho-cresyl Phosphate Through Regulation the Autophagy Flux in Hens. Neurochem Res 40, 2374–2382 (2015). https://doi.org/10.1007/s11064-015-1725-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1725-8