Abstract
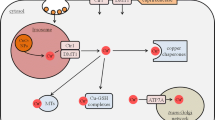
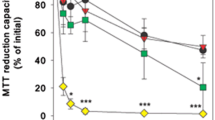
Copper oxide nanoparticles (CuO-NPs) are frequently used for industrial or medical applications and are known for their high toxic potential. As little is known so far on the consequences of an exposure of brain cells to such particles, we applied CuO-NPs to cultured primary rat astrocytes and investigated whether such particles affect cell viability and alter their metabolic properties. Astrocytes efficiently accumulated CuO-NPs in a time- and concentration-dependent manner. The cells remained viable during a 24 h incubation with 100 µM copper in the form of CuO-NPs, while higher concentrations of CuO-NPs severely compromised the cell viability. Astrocytes that were exposed for 24 h to 100 µM CuO-NPs showed significantly enhanced extracellular lactate concentrations and increased cellular levels of glutathione and metallothioneins. The CuO-NP-induced increase in lactate release and metallothionein content were prevented by the presence of the membrane-permeable copper chelator tetrathiomolybdate, while this chelator increased already in the absence of CuO-NPs the cellular glutathione content. After removal of the CuO-NPs following a 24 h pre-incubation with 100 µM CuO-NPs, astrocytes maintained during a further 6 h incubation an elevated glycolytic lactate release and exported significantly more glutathione than control cells that had been pre-incubated without CuO-NPs. These data suggest that copper ions which are liberated from internalized CuO-NPs stimulate glycolytic flux as well as the synthesis of glutathione and metallothioneins in cultured viable astrocytes.





Similar content being viewed by others
References
Hanagata N, Zhuang F, Connolly S, Li J, Ogawa N, Xu M (2011) Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano 5:9326–9338
Li CW, Ciston J, Kanan MW (2014) Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508:504–507
Patil PR, Krishnamurthy VN, Joshi SS (2008) Effect of nano-copper oxide and copper chromite on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech 33:266–270
Rubilar O, Rai M, Tortella G, Diez MC, Seabra AB, Duran N (2013) Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol Lett 35:1365–1375
Ahmad Z, Vargas-Reus MA, Bakhshi R, Ryan F, Ren GG, Oktar F, Allaker RP (2012) Antimicrobial properties of electrically formed elastomeric polyurethane–copper oxide nanocomposites for medical and dental applications. Methods Enzymol 509:87–99
Dankovich TA, Smith JA (2014) Incorporation of copper nanoparticles into paper for point-of-use water purification. Water Res 63C:245–251
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Balkhyour MA, Goknil MK (2010) Total fume and metal concentrations during welding in selected factories in Jeddah, Saudi Arabia. Int J Environ Res Public Health 7:2978–2987
Szymczak W, Menzel N, Keck L (2007) Emission of ultrafine copper particles by universal motors controlled by phase angle modulation. J Aerosol Sci 38:520–531
Karlsson HL, Cronholm P, Gustafsson J, Moller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21:1726–1732
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB, Alhadlaq HA (2010) Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun 396:578–583
Xu J, Li Z, Xu P, Xiao L, Yang Z (2013) Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. Arch Toxicol 87:1067–1073
Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA (2013) Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol 32:296–307
Laha D, Pramanik A, Maity J, Mukherjee A, Pramanik P, Laskar A, Karmakar P (2014) Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim Biophys Acta 1840:1–9
Siddiqui MA, Alhadlaq HA, Ahmad J, Al-Khedhairy AA, Musarrat J, Ahamed M (2013) Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One 8:e69534
Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22:64–75
Bulcke F, Thiel K, Dringen R (2014) Uptake and toxicity of copper oxide nanoparticles in cultured primary brain astrocytes. Nanotoxicology 8:775–785
An L, Liu S, Yang Z, Zhang T (2012) Cognitive impairment in rats induced by nano-CuO and its possible mechanisms. Toxicol Lett 213:220–227
Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A (2002) Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A 65:1513–1530
Lockman PR, Koziara JM, Mumper RJ, Allen DD (2004) Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target 12:635–641
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445
Sharma HS, Sharma A (2012) Neurotoxicity of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets 11:65–80
Yim YS, Choi JS, Kim GT, Kim CH, Shin TH, Kim DG, Cheon J (2012) A facile approach for the delivery of inorganic nanoparticles into the brain by passing through the blood-brain barrier (BBB). Chem Commun (Camb) 48:61–63
Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS (2012) Demonstration of an olfactory bulb–brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci 48:464–471
Karmakar A, Zhang Q, Zhang Y (2014) Neurotoxicity of nanoscale materials. J Food Drug Anal 22:147–160
Liu Y, Gao Y, Liu Y, Li B, Chen C, Wu G (2014) Oxidative stress and acute changes in murine brain tissues after nasal instillation of copper particles with different sizes. J Nanosci Nanotechnol 14:4534–4540
Liu Y, Gao Y, Zhang L, Wang T, Wang J, Jiao F, Li W, Liu Y, Li Y, Li B, Chai Z, Wu G, Chen C (2009) Potential health impact on mice after nasal instillation of nano-sized copper particles and their translocation in mice. J Nanosci Nanotechnol 9:6335–6343
Cupaioli FA, Zucca FA, Boraschi D, Zecca L (2014) Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol 119–120C:20–38
López-Hidalgo M, Schummers J (2014) Cortical maps: a role for astrocytes? Curr Opin Neurobiol 24:176–189
Verkhratsky A, Nedergaard M, Hertz L (2014) Why are astrocytes important? Neurochem Res (in press)
Scheiber IF, Dringen R (2013) Astrocyte functions in the copper homeostasis of the brain. Neurochem Int 62:556–565
Hohnholt MC, Geppert M, Luther EM, Petters C, Bulcke F, Dringen R (2013) Handling of iron oxide and silver nanoparticles by astrocytes. Neurochem Res 38:227–239
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57
Scheiber IF, Mercer JF, Dringen R (2010) Copper accumulation by cultured astrocytes. Neurochem Int 56:451–460
Scheiber IF, Schmidt MM, Dringen R (2012) Copper export from cultured astrocytes. Neurochem Int 60:292–300
Scheiber IF, Dringen R (2011) Copper-treatment increases the cellular GSH content and accelerates GSH export from cultured rat astrocytes. Neurosci Lett 498:42–46
Scheiber IF, Dringen R (2011) Copper accelerates glycolytic flux in cultured astrocytes. Neurochem Res 36:894–903
Petters C, Irrsack E, Koch M, Dringen R (2014) Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem Res 39:1648–1660
Hamprecht B, Löffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of rat astrocytes and neurons as model systems to study metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen H (eds) Neuromethods 90: brain energy metabolism. Springer, Heidelberg, pp 45–72
Petters C, Dringen R (2014) Comparison of primary and secondary rat atrocyte cultures regarding glucose and glutathione metabolism and the accumulation of iron oxide nanoparticles. Neurochem Res 39:46–58
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Protoc 2:223–228
Liddell JR, Zwingmann C, Schmidt MM, Thiessen A, Leibfritz D, Robinson SR, Dringen R (2009) Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. J Neurosci Res 87:2696–2708
Hirrlinger J, Dringen R (2005) Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol 400:395–409
Hohnholt MC, Geppert M, Dringen R (2011) Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater 7:3946–3954
Luther E, Schmidt M, Diendorf J, Epple M, Dringen R (2012) Upregulation of metallothioneins after exposure of cultured primary astrocytes to silver nanoparticles. Neurochem Res 37:1639–1648
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 22:375101
Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M (2014) Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health (in press)
Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, Wallinder IO, Moller L (2013) Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small 9:970–982
Di Bucchianico S, Fabbrizi MR, Misra SK, Valsami-Jones E, Berhanu D, Reip P, Bergamaschi E, Migliore L (2013) Multiple cytotoxic and genotoxic effects induced in vitro by differently shaped copper oxide nanomaterials. Mutagenesis 28:287–299
Wang Z, von dem Bussche A, Kabadi PK, Kane AB, Hurt RH (2013) Biological and environmental transformations of copper-based nanomaterials. ACS Nano 7:8715–8727
Geppert M, Petters C, Thiel K, Dringen R (2013) The presence of serum alters the properties of iron oxide nanoparticles and lowers their accumulation by cultured brain astrocytes. J Nanopart Res 15:1349–1364
Lamkowsky MC, Geppert M, Schmidt MM, Dringen R (2012) Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J Biomed Mater Res A 100A:323–334
Cuillel M, Chevallet M, Charbonnier P, Fauquant C, Pignot-Paintrand I, Arnaud J, Cassio D, Michaud-Soret I, Mintz E (2014) Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. Nanoscale 6:1707–1715
Wang Z, Li N, Zhao J, White JC, Qu P, Xing B (2012) CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol 25:1512–1521
Clarke NJ, Laurie SH (1982) The copper–molybdenium antagonism in ruminants. II: interactions of thiomolybdates with copper(II) in aqueous media. Inorg Chim Acta 66:L35–L38
Geppert M, Hohnholt MC, Nurnberger S, Dringen R (2012) Ferritin up-regulation and transient ROS production in cultured brain astrocytes after loading with iron oxide nanoparticles. Acta Biomater 8:3832–3839
Czachor JD, Cherian MG, Koropatnick J (2002) Reduction of copper and metallothionein in toxic milk mice by tetrathiomolybdate, but not deferiprone. J Inorg Biochem 88:213–222
Nemec AA, Leikauf GD, Pitt BR, Wasserloos KJ, Barchowsky A (2009) Nickel mobilizes intracellular zinc to induce metallothionein in human airway epithelial cells. Am J Respir Cell Mol Biol 41:69–75
Parat M-O, Richard M-J, Meplan C, Favier A, Béani J-C (1999) Impairment of cultured cell proliferation and metallothionein expression by metal chelator NNN′ N′-tetrakis-(2-pyridylmethyl) ethylene diamine. Biol Trace Elem Res 70:51–68
Sagara J, Makino N, Bannai S (1996) Glutathione efflux from cultured astrocytes. J Neurochem 66:1876–1881
Węgrzynowicz M, Hilgier W, Dybel A, Oja SS, Saransaari P, Albrecht J (2007) Upregulation of cerebral cortical glutathione synthesis by ammonia in vivo and in cultured glial cells: the role of cystine uptake. Neurochem Int 50:883–889
Tulpule K, Schmidt MM, Boecker K, Goldbaum O, Richter-Landsberg C, Dringen R (2012) Formaldehyde induces rapid glutathione export from viable oligodendroglial OLN-93 cells. Neurochem Int 61:1302–1313
Hirrlinger J, König J, Keppler D, Lindenau J, Schulz JB, Dringen R (2001) The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem 76:627–636
Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97:373–384
Chao PY, Allen KG (1992) Glutathione production in copper-deficient isolated rat hepatocytes. Free Radic Biol Med 12:145–150
Panneerselvam SR, Govindasamy S (2004) Effect of sodium molybdate on the status of lipids, lipid peroxidation and antioxidant systems in alloxan-induced diabetic rats. Clin Chim Acta 345:93–98
Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Bouzier-Sore AK, Pellerin L (2013) Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci 7:179
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bulcke, F., Dringen, R. Copper Oxide Nanoparticles Stimulate Glycolytic Flux and Increase the Cellular Contents of Glutathione and Metallothioneins in Cultured Astrocytes. Neurochem Res 40, 15–26 (2015). https://doi.org/10.1007/s11064-014-1458-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1458-0