Abstract
Purpose
To assess the impact of individual surgeon experience on overall survival (OS), extent of resection (EOR) and surgery-related morbidity in elderly patients with glioblastoma (GBM), we performed a retrospective case-by-case analysis.
Methods
GBM patients aged ≥ 65 years who underwent tumor resection at two academic centers were analyzed. The experience of each neurosurgeon was quantified in three ways: (1) total number of previously performed glioma surgeries (lifetime experience); (2) number of surgeries performed in the previous five years (medium-term experience) and (3) in the last two years (short-term experience). Surgeon experience data was correlated with survival (OS) and surrogate parameters for surgical quality (EOR, morbidity).
Results
198 GBM patients (median age 73.0 years, median preoperative KPS 80, IDH-wildtype status 96.5%) were included. Median OS was 10.0 months (95% CI 8.0–12.0); median EOR was 89.4%. Surgery-related morbidity affected 19.7% patients. No correlations of lifetime surgeon experience with OS (P = .693), EOR (P = .693), and surgery-related morbidity (P = .435) were identified. Adjuvant therapy was associated with improved OS (P < .001); patients with surgery-related morbidity were less likely to receive adjuvant treatment (P = .002). In multivariable testing, adjuvant therapy (P < .001; HR = 0.064, 95%CI 0.028–0.144) remained the only significant predictor for improved OS.
Conclusion
Less experienced neurosurgeons achieve similar surgical results and outcome in elderly GBM patients within the setting of academic teaching hospitals. Adjuvant treatment and avoidance of surgery-related morbidity are crucial for generating a treatment benefit for this cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) CNS World Health Organization (WHO) grade 4 is the most common, malignant intrinsic brain tumor in adult patients [1, 2] with a reported median age at initial diagnosis of approximately 65 years [2]. Elderly high-grade glioma patients show a worse overall survival (OS) compared to younger patients, with reduced ability to tolerate therapeutic interventions and higher rates of unfavorable biomarker status [3, 4]. Only moderate effects of gross total resection (GTR) – especially compared to biopsy alone – on OS have been demonstrated in patients older than 65 years [5,6,7,8,9,10]. Considering this background any surgery-related morbidity in elderly GBM patients might mitigate the potential benefits of aggressive surgical treatment [8, 10,11,12,13,14,15].
Prior studies have shown that a center’s higher caseload/surgeon volume for surgically treated oncological patients leads to improved outcome [16, 17], an association that has been substantiated for several neurosurgical procedures [18,19,20,21,22]. Two different studies have previously reported a favorable outcome for GBM patients if surgery is performed by specialist neurooncology neurosurgeons [23] and if patients are treated at academic/high-volume centers [23, 24]. Here we evaluated the impact of surgeon experience on elderly (i.e. age ≥ 65 years at diagnosis) GBM patients’ outcome after microsurgical tumor resection.
We hypothesized that greater individual surgeon’s experience leads to (1) improved OS, (2) improved EOR, and (3) decreased surgery-related morbidity/mortality.
Methods
Patient selection and histopathology
We identified GBM patients with an age ≥ 65 years at the date of initial surgery, who had undergone microsurgical resection as primary treatment at two university hospitals between 02/2012 and 12/2016 (Center A) and 09/2006 to 06/2021 (Center B). Exclusion criteria were defined as emergency surgery, biopsy only, palliative care only as well as patients with known secondary GBMs. The two hospitals have a combined catchment area of approximately 2,250,000 inhabitants. Annual caseloads of glioma surgeries were approximated to be ~ 120 (Center A) and ~ 50 (Center B).
Histopathological grading was performed according to the WHO classification of tumors of the central nervous system valid at the time of diagnosis [25, 26]. Clinical data extraction was performed from electronic medical records and included patient characteristics such as age, sex, preoperative Karnofsky Performance Scale (KPS), tumor location and leading symptoms.
All patients/legal guardians gave written informed consent prior to all surgical procedures and adjuvant treatments. The State Review Board approval was obtained for the retrospective GBM databases this study was conducted upon (IRB-Number 415-E/2247/2-2017).
Course of treatment, follow-up, morbidity and mortality
Fluorescence-guided surgeries with 5-aminolevulinic acid (5-ALA) as well as intraoperative ultrasound and neuronavigation were routinely used at both centers; one center had additional access to intraoperative MRI for selected cases (Center A).
Postoperatively, patients were evaluated at the neurosurgical outpatient clinic, usually within two to four weeks, and underwent magnetic resonance imaging (MRI) at 3-months intervals over the course of follow-up. All adjuvant treatment and surgery-associated morbidity/mortality were recorded; surgery-related morbidity and mortality were defined as death within 30 days after surgery, new or worsening preoperative neurologic deficits/epilepsy, symptomatic intracranial hemorrhage, pulmonary embolism, organic psychosyndrome (OPS), cerebrospinal fluid fistulas requiring revision surgery and local/systemic infections. OS was calculated until last clinical follow-up or death; the date of last follow-up was November 30th, 2021.
Surgeon experience data
We determined all corresponding surgical teams for the recorded tumor resections. Each surgical team consisted of two neurosurgeons, at least one of whom held a neurosurgical board certification. For each lead surgeon, we calculated the total number of previously performed cranial microsurgical glioma resections (lifetime experience), the number of surgeries performed in the last five years (medium term) and two years (short term) for each index surgery. If we were unable to obtain this information, these cases were excluded.
Imaging analysis
For the majority of cases, preoperative and early postoperative (≤ 72 h after surgery) magnetic resonance imaging (MRI) data were available. All available pre- and immediate postoperative MRIs were centrally re-evaluated to determine the EOR by members of the Institute of Neuroradiology (LM, JS) blinded to the patients’ clinical outcome and surgical teams (Supplement 1). The difficulty level of all performed surgeries was assessed by the Milan Complexity Scale (MCS), i.e. with regard to major brain vessel manipulation, posterior fossa location, cranial nerve manipulation, eloquence and tumor size [27].
Statistical analysis
Survival analyses were performed using the Kaplan-Meier method. Surgeon experience for all three time spans (i.e. lifetime, medium- and short-term experiences) was correlated with OS, EOR and surgery-related morbidity and mortality (Supplement 1). Given that it has been reported that GBM outcome improved during the time frame of this study [3], we performed separate outcome analyses for two dichotomized periods (i.e. period 1 (≤ 2013) and period 2 (2014 and onwards) in an effort to rule out the surgery date itself as a potential confounding factor.
Results
198 GBM patients (112 males) met inclusion criteria. Median age at tumor resection was 73.0 years (range 65–88), median preoperative KPS was 80 (range 40–100). Most common symptoms leading to diagnosis were epileptic seizures (n = 35/198), motor deficits (n = 30/198) and headache (n = 25/198). The most common tumor locations were the temporal (n = 65) and frontal lobe (n = 55). Histopathological analyses revealed an IDH1/2 wildtype status in 191/195 (97.9%) tumors (Table 1).
Treatment, clinical outcome and extent of resection
Median OS was 10.0 months (95%CI 8.0–12.0), the median EOR of 89.4% (range 12.7–100). Gross total resection (EOR ≥ 95%) was recorded for 57/171 (33.3%) patients. Most patients 154/198 (77.8%) received adjuvant treatment most frequently consisting of concomitant radiochemotherapy (RTx/CTx) followed by adjuvant temozolomide [28] (Table 2). Adjuvant therapy correlated with improved median OS of 15.0 (95%CI 11.8–18.2) vs. 2.0 (95%CI 0.0–4.2) months (p < .001) (Fig. 1A).
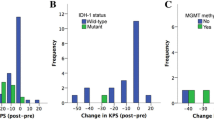
Surgery-related morbidity and mortality was seen in 39/198 (19.7%) including 8/198 (4.0%) deaths. The most frequent surgery-related morbidity consisted of either new neurological deficits (n = 37), most commonly a new hemiparesis (n = 12) or worsening of a preexisting hemiparesis (n = 7), symptomatic postoperative hemorrhage (n = 13), and OPS (n = 12). Patients with surgery-related morbidity had worse OS (median 10.0 (95%CI 7.0–13.0) vs. 11.0 (95%CI 7.9–14.1) months; mean 10.4 ± 1.2 vs. 17.9 ± 2.0 months) not statistically significant (P = .051). Patients with surgery-related morbidity were less likely to receive adjuvant treatment (P = .002); 35.9% of patients with surgery-related morbidity did not receive adjuvant treatment vs. 12.9% of cases without surgery-related morbidity. Thus, surgery-related morbidity was a significant predictor for withholding further treatment (P = .019). Accordingly, a higher postoperative disability as assessed by modified Rankin Scale correlated with worse OS (p ≤ .001). Higher preoperative KPS (≥ 70 vs. < 70; P = .040) and younger age (≤ 73 years; P = .030) were associated with improved OS (Fig. 1B C). Tumor volume (P = .146) and EOR (P = .469) did not correlate with OS. MGMT-promoter methylation did not correlate with improved OS (P = .632) in our patient cohort; furthermore, MGMT-promoter methylation did not associate with surgery-related morbidity (P = .599) and performance of adjuvant treatment (P = .399).
Additional analyses showed that there was a significant difference with regard to median EOR between centers (86.8% (IQR 62.7–95.2) vs. 90.5% (IQR 81.5–97.2); p = .041) but also with regard to the estimated median MCS (4.0 (IQR 2.0–4.0) vs. 2.0 (IQR 1.0–4.0); P < .001) (Table 2). No significant differences with regard to surgery-related morbidity frequencies (P = .846), PFS (P = .669), and OS (P = .143) between the two centers were noted in univariate analyses (Table 1). Differences in OS between periods 1 and 2 (9.0 (95%CI 7.2–10.8) vs.13.0 (95%CI 9.4–16.6) months were not statistically significant (P = .231). Also, adjuvant treatment frequencies between inclusion periods 1 (77.9%) and period 2 (77.7%) (P = .220) remained similar.
Surgeon experience data
We identified 37 different neurosurgeons who had performed the analyzed glioma surgeries in this series; 18 (48.7%) at Center A and 19 (51.4%) at Center B (Table 3), in 172/198 (86.9%) cases the lead neurosurgeon held a board certification at the time of tumor resection. On average 61.5 ± 54.6 (median 50, range 0–232) glioma surgeries had been performed before the index surgery by the lead surgeon. The respective average numbers for medium-term and short-term experience were 30.9 ± 22.8 (median 30, range 0–96) and 13.8 ± 9.8 (median 13, range 0–41).
Impact of surgeon experience on outcome parameters
Lifetime surgical experience as a continuous variable did not show a significant correlation with OS (P = .693), EOR (P = .693), or occurrence of surgery-related morbidity (P = .435). These results were corroborated by the respective correlation analyses for medium-term (OS (P = .386), EOR (P = .542) and surgery-related morbidity (P = .530)) as well as short-term (OS (P = .499), EOR (P = .555) and surgery-related morbidity (P = .450)) experiences.
Similarly, correlation analyses with the dichotomised data did not demonstrate any significant correlation of lifetime experience (OS (P = .909), EOR (P = .545) and surgery-related morbidity (P = .132)), medium-term experience (OS (P = .111), EOR (P = .415) and surgery-related morbidity (P = .234)) and short-term experience (OS (P = .241), EOR (P = .356), surgery-related morbidity (P = .771)) (Fig. 1D, E and F and Table 3).
In a multivariable cox regression model, adjuvant therapy remained the only significant predictor for improved OS (P < .001; HR = 0.064, 95%CI 0.028–0.144), neither lifetime surgeon experience nor medium-term and short-term experiences correlated significantly with patient survival (Table 4).
Further in-depth analyses showed no correlation between MCS and surgery-related morbidity (P = .465) or the surgeons’ experience (P = .132); thus more experienced surgeons did not operate tumors of an estimated higher difficulty. Furthermore, the prognostic factors (i.e. patient age, KPS, adjuvant treatment) for improved OS were equally distributed within surgeon experience median split subgroups (Supplement 2).
Discussion
The key findings of our study were that (1) less experienced neurosurgeons achieve similar surgical results with regard to clinical and radiological outcome in elderly GBM, IDH-wildtype patients, and (2) avoidance of surgery-related morbidity and performance of adjuvant treatment after tumor resection are crucial for generating a treatment benefit.
Compared to other studies, which often use the total provider volume and/or the annual surgeon volume as a surrogate parameter to evaluate volume-outcome associations in neurosurgery [20, 21], [29,30,31], we conducted a case-by-case analysis for each individual surgeon’s experience at the time of surgery. Our main hypothesis, that greater surgeon experience provides better clinical and surgical outcome, was falsified. This was consistent concerning OS, EOR and surgery-related morbidity. Contrary to our findings, in a study of younger patients (median age 54 to 57 years) with high-grade gliomas WHO grade 3 and 4, an improved OS and a lower 30-day mortality has been shown for specialist surgical neurooncologists compared to general neurosurgeons [23]. Thus, a positive effect of a surgeon’s experience in a younger cohort with supposedly more IDH-mutant gliomas seems to be evident [23]. The minor influence of surgeon volume on patient outcome in our cohort, most likely reflects the fatal course of GBM, IDH-wildtype in patients of higher age. This leads to the assumption that in elderly GBM, IDH-wildtype patients the efficacy of surgery itself is possibly limited to facilitate adjuvant therapy. This aspect underscores that a surgeon´s individual experience only plays a minor role for outcome in elderly GBM patients, because surgery itself – no matter how extensive/well-performed – has no significant influence on clinical outcome. Of note, MGMT-promoter-methylation status did not correlate with OS and performance of adjuvant treatment in our data. This somewhat contradictive aspect regarding MGMT status and survival in elderly GBM patients has also been recorded before [8].
On the other hand, no objectifiable difference in surgical quality between experienced and less experienced surgeons could be documented since we found no difference in EOR, surgery-related morbidity and mortality analyses with regard to surgeon experience. Other studies on surgeon experience in brain tumor patients have shown a reduction in 30-day mortality after brain tumor resection by more experienced surgeons [31], and improved early postoperative outcome measures for annual and five-year interval caseloads [18] whereas similar results could be achieved by less experienced surgeons compared to high-volume surgeons in very old meningioma patients [19]. In our cohort, any surgery which was performed by a less experienced surgeon was supervised by an assisting attending. This academic educational system seems to offer stable surgical quality as measured in EOR and complication rates, even in absence of an experienced lead surgeon. Although we do not believe that GBM surgery in elderly patients should be left to inexperienced residents, we did find reasonable evidence, that under experienced guidance less experienced surgeons can perform these surgeries without compromising clinical outcome.
Even though patients older than 65 years represent about half of all GBM patients [2], patients older than 70 years were excluded from some of the most important therapy defining studies [28], leaving the treatment of elderly GBM patients to case-by-case decisions. Thus, a decent approach to define appropriate treatment algorithms is necessary and first guidelines on the topic have been published [32, 33], focusing on the efficacy of cytoreduction apart from GTR only paradigms. Although a benefit of GTR in GBM patients has been confirmed by many studies [34,35,36,37], data on the impact of EOR on outcome in elderly (≥ 65), often fragile patients are scarce and less specific, but favor resection over biopsy only protocols [4, 8, 10, 12, 38, 39]. A survival benefit for any microsurgical glioma surgery compared to biopsy only in IDH-wildtype GBM patients – regardless of the extent of resection – has been reported [39, 40] as well as a survival benefit for GTR compared to partial/subtotal resection and biopsy only in a recent study with a large cohort of GBM patients older than 65 years [8]. Since GBM patients who only underwent biopsy were excluded from our study, we cannot draw a reasonable conclusion in favor of glioma resection in elderly GBM patients. However, according to our data, the extent of resection itself seems less critical in this specific patient subgroup. Thus, in elderly GBM patients the avoidance of complications to enable adjuvant treatment is probably more important than maximizing EOR at any cost. This point of view is reflected by several other authors [11, 15, 33, 41] and substantiated here for elderly, supposedly more fragile GBM, IDH-wildtype patients, since the overwhelming influence of maximized EOR has been shown to be of prognostic relevance in multiple prior studies on GBM in more age-balanced cohorts [34,35,36]. Moreover, it can be stated that the avoidance of complications adds to the quality of life in the last months of an elderly GBM patient – which is obviously paramount.
In summary, the two highly significant findings of this study with regard to clinical outcome are, firstly, the importance of adjuvant therapy for survival and, secondly, avoidance of surgery-related morbidity. This aspect is in line with prior data, in which a survival benefit for GBM patients treated at academic and high-volume centers compared to low volume/non-academic facilities could be shown [24]. Thus, the primary importance is not the experience level of the surgeons, but the impact of adjuvant treatment, which might be more utilized in academic and high-volume facilities.
Limitations
In order to most reliably and objectively assess the effect of surgeon experience on the chosen outcome parameters, we opted for a case-by-case analysis approach. Nonetheless, it remains an immanent limitation that no two surgeries are alike. In the best effort to rule out potential bias factors, we retrospectively scored the assumed complexity of the surgery by the MCS and checked for any imbalances of known prognostic factors for improved OS within the analyzed subgroups. In addition, due to the limited case number there is potential risk for type 2 errors.
Furthermore, all surgeries were supervised by at least one board-certified neurosurgeon and the degree of help received by a younger colleague during the tumor resection could not be retrospectively verified by the available surgical reports. Board certification of the lead surgeon per se did not correlate with patient outcome (data not shown).
Conclusion
According to our data, surgical resection of GBM, IDH-wildtype in patients aged ≥ 65 years may be safely performed by less experienced surgeons in the setting of an academic teaching hospital – under supervision of more experienced neurosurgeons – without compromising clinical outcome parameters. In this often fragile patient cohort, avoidance of postoperative morbidity and performance of adjuvant treatment remain most significant adjustable predictors for improved clinical outcome and should be the main focus of any surgical intervention. The observed limited influence of surgeon volume on patient outcome most likely reflects the fatal course of GBM, IDH-wildtype in patients of higher age.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- CTx:
-
Chemotherapy
- EOR:
-
Extent of resection
- GBM:
-
Glioblastoma
- IDH:
-
Isocitrate dehydrogenase
- KPS:
-
Karnofsky performance scale
- MCS:
-
Milan complexity scale
- MGMT:
-
O(6)-methylguanine-DNA methyltransferase
- MRI:
-
Magnetic resonance imaging
- OPS:
-
Organic psychosyndrome
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RANO:
-
Response assessment in neuro-oncology
- RTx:
-
Radiotherapy
- TERT:
-
Telomerase reverse transcriptase
- TMZ:
-
Temozolomide
- WHO:
-
World health organisation
References
Miller KD et al (2021) Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. https://doi.org/10.3322/caac.21693
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. https://doi.org/10.1093/neuonc/noab200
Korja M et al (2019) Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol. https://doi.org/10.1093/neuonc/noy164
Pirkkalainen J-M, Jääskeläinen A-S, Halonen P (2022) Retrospective single-center study on elderly patients with glioblastoma between 2014 and 2018 evaluating the effect of age and performance status on survival. Neurooncol Pract. https://doi.org/10.1093/nop/npac008
Barak T et al (2021) Surgical strategies for older patients with glioblastoma. J Neurooncol. https://doi.org/10.1007/s11060-021-03862-z
Barnholtz-Sloan JS et al (2008) Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. https://doi.org/10.3171/JNS/2008/108/4/0642
Fabbro-Peray P et al (2019) Association of patterns of care, prognostic factors, and use of radiotherapy–temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a french national population-based study. J Neurooncol. https://doi.org/10.1007/s11060-018-03065-z
Heiland DH et al (2018) One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neurooncol. doi: https://doi.org/10.1007/s11060-018-2964-8
Osawa T, Tosaka M, Horiguchi K, Sugawara K, Yokoo H, Yoshimoto Y (2021) Elderly patients aged over 75 years with glioblastoma: preoperative status and surgical strategies. Interdiscip Neurosurg. https://doi.org/10.1016/j.inat.2021.101127
Lopez-Rivera V et al (2021) Extent of resection and survival outcomes of geriatric patients with glioblastoma: is there benefit from aggressive surgery? Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2021.106474
Karsy M et al (2018) Surgical treatment of glioblastoma in the elderly: the impact of complications. J Neurooncol. https://doi.org/10.1007/s11060-018-2777-9
Schwartz C et al (2020) Risks and benefits of Glioblastoma Resection in older adults: a retrospective austrian Multicenter Study. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.09.097
C. K.L., et al (2011) Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. J Neurosurg 114:587
Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE (2009) Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center experience (1997–2007). Cancer. https://doi.org/10.1002/cncr.24413
Wick A et al (2018) Glioblastoma in elderly patients: solid conclusions built on shifting sand? Neuro Oncol. https://doi.org/10.1093/neuonc/nox133
Fischer C et al (2017) Volume-outcome revisited: the effect of hospital and surgeon volumes on multiple outcome measures in oesophago-gastric cancer surgery. PLoS ONE. https://doi.org/10.1371/journal.pone.0183955
Bedi HK, Jedrzejko N, Nguyen A, Aspinall SR, Wiseman SM (2021) Thyroid and parathyroid surgeon case volume influences patient outcomes: a systematic review. Surg Oncol. https://doi.org/10.1016/j.suronc.2021.101550
Ramakrishna R, Hsu WC, Mao J, Sedrakyan A (2018) Surgeon Annual and cumulative volumes predict early postoperative Outcomes after Brain Tumor Resection. World Neurosurg. https://doi.org/10.1016/j.wneu.2018.02.172
Rautalin I, Schwartz C, Niemelä M, Korja M (2021) Effect of surgeon experience on surgical outcome of 80-year-old or older intracranial meningioma patients. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.12.166
Davies JM, Lawton MT (2017) Improved outcomes for patients with cerebrovascular malformations at high-volume centers: the impact of surgeon and hospital volume in the United States, 2000–2009. J Neurosurg. https://doi.org/10.3171/2016.7.JNS15925
Trinh VT, Davies JM, Berger MS (2015) Surgery for primary supratentorial brain tumors in the United States, 2000–2009: effect of provider and hospital caseload on complication rates. J Neurosurg. https://doi.org/10.3171/2014.9.JNS131648
Curry WT, McDermott MW, Carter BS, Barker FG (2005) Craniotomy for meningioma in the United States between 1988 and 2000: decreasing rate of mortality and the effect of provider caseload. J Neurosurg. https://doi.org/10.3171/jns.2005.102.6.0977
Khan UA et al (2015) Treatment by specialist surgical neurooncologists improves survival times for patients with malignant glioma. J Neurosurg. https://doi.org/10.3171/2014.10.JNS132057
Zhu P, Du XL, Zhu JJ, Esquenazi Y (2020) Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the national cancer database. J Neurosurg. https://doi.org/10.3171/2018.10.JNS182247
Louis DN et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. https://doi.org/10.1007/s00401-007-0243-4
Louis DN et al (2016) The 2016 World Health Organization classification of tumors of the Central Nervous System: a summary. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1545-1
Ferroli P et al (2015) Predicting functional impairment in brain tumor surgery: the big five and the Milan Complexity Scale. Neurosurg Focus. https://doi.org/10.3171/2015.9.FOCUS15339
Stupp R et al (2005) Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New Engl J Med. https://doi.org/10.1056/nejmoa043330
Kalkanis SN et al (2003) Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. https://doi.org/10.1227/01.NEU.0000065129.25359.EE
Cowan JA et al (2003) The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery. https://doi.org/10.1097/00006123-200301000-00005
Williams M, Treasure P, Greenberg D, Brodbelt A, Collins P (2016) Surgeon volume and 30 day mortality for brain tumours in England. Br J Cancer. https://doi.org/10.1038/bjc.2016.317
Domino JS, Ormond DR, Germano IM, Sami M, Ryken TC, Olson JJ (2020) Cytoreductive surgery in the management of newly diagnosed glioblastoma in adults: a systematic review and evidence-based clinical practice guideline update. J Neurooncol. https://doi.org/10.1007/s11060-020-03606-5
Laperriere N et al (2013) Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. https://doi.org/10.1016/j.ctrv.2012.05.008
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas: clinical article. J Neurosurg. https://doi.org/10.3171/2011.2.JNS10998
McGirt MJ et al (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma: clinical article. J Neurosurg. https://doi.org/10.3171/2008.4.17536
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery. https://doi.org/10.1227/01.neu.0000318159.21731.cf
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(06)70665-9
Noorbakhsh A et al (2014) Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. J Neurosurg. https://doi.org/10.3171/2013.9.JNS13877
Chaichana KL et al (2011) Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. https://doi.org/10.1245/s10434-010-1242-6
Hallaert G et al (2020) Partial resection offers an overall survival benefit over biopsy in MGMT-unmethylated IDH-wildtype glioblastoma patients. Surg Oncol. https://doi.org/10.1016/j.suronc.2020.10.016
Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J (2003) Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-002-1030-6
Funding
Open access funding provided by Paracelsus Medical University. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript
Author information
Authors and Affiliations
Contributions
JPP, CS and LM contributed to the study conception and design. Material preparation and data collection were performed by JPP, CS, LM, MD, BL, SE, JS and HS Statistical Analysis was performed bei LM, JPP and CS The first draft of the manuscript was written by JPP, CS, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Figures and Tables were prepared by JPP, LM and CS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. No study-specific funding was received.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval for the underlying database for “Treatment of glioblastoma multiforme WHO IV in the elderly” was granted by the Ethics Committee of the Country of Salzburg (25.10.2017/No 415-E/2247/2-2017).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pöppe, J.P., Machegger, L., Steinbacher, J. et al. Surgeon experience in glioblastoma surgery of the elderly—a multicenter, retrospective cohort study. J Neurooncol 161, 563–572 (2023). https://doi.org/10.1007/s11060-023-04252-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04252-3